Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.27 n.1 Mendoza jan./abr. 2003
Antibacterial activity of lactose-binding lectins from Bufo arenarum skin
Alicia Sánchez Riera, Adriana Daud, Adriana Gallo, Susana Genta, Manuel Aybar, and Sara Sánchez
Departamento de Biología del Desarrollo, Instituto Superior de Investigaciones Biológicas (INSIBIO) y Universidad Nacional de Tucumán (UNT). Chacabuco 461, (4000) San Miguel de Tucumán, Tucumán, Argentina.
Address correspondence to: Dra. Alicia Sánchez Riera. Departamento de Biología del Desarrollo, INSIBIO (CONICET-UNT). Chacabuco 461. (4000) San Miguel de Tucumán, Tucumán, ARGENTINA. Fax: (+54-381) 424 8025. E-mail: sariera@unt.edu.ar
Key words: skin, lectin, amphibian, antibacterial activity.
ABSTRACT: Amphibians respond to microbial infection through cellular and humoral defense mechanisms such as antimicrobial protein secretion. Most humoral defense proteins are synthetized in the skin. In this study we isolated two b-galactoside-binding lectins with molecular weights of 50 and 56 KDa from the skin of Bufo arenarum. These lectins have significant hemagglutination activity against trypsinized rabbit erythrocytes, which was inhibited by galactose-containing saccharides. They are water-soluble and independent of the presence of calcium. The antimicrobial analysis for each lectin was performed. At mmolar concentration lectins show strong bacteriostatic activity against Gram negative bacteria (Escherichia coli K12 4100 and wild strains of Escherichia coli and Proteus morganii) and Gram positive bacteria (Enterococcus faecalis). The antibacterial activity of these lectins may provide an effective defense against invading microbes in the amphibian Bufo arenarum.
Introduction
Organisms ward of microbial infection through well described cellular and humoral mechanisms. A little studied branch of the host defense system involves the production of antimicrobial substances. In animals, antimicrobial molecules are produced in the skin, in simple epithelial tissues and in acute inflammatory cells, where they act as a first-line defense against invasion, thus supplementing the host humoral and cellular immune system.
In amphibians, skin secretions contain many biologically active compounds, such as biogenic amines (Erspamer, 1971), complex alkaloids (Erspamer, 1993), hormones noxious to predators (Kuchler et al., 1990), antibiotic peptides (Clarck et al., 1994) and lectins (Marschal et al., 1992).
The latter are a group of various proteins characterized by their ability to bind carbohydrates with considerable specifity. They are found in organisms ranging from viruses to plants and animals.
Over 60% of the lectins reported so far are galactose-specific, probably because galactose is an important recognition saccharide, especially in higher organisms. In terms of apparent molecular mass, there are two types of galactose-binding lectins: a small one (approximately 14 kDa) and a large one (approximately 30 kDa). Partial and complete primary structures of the small type metal independent lectins have been reported for mouse (Joubert et al., 1988; Wells and Mallucci, 1991), chicken (Sakakura et al., 1990; Akimoto et al., 1995), hamster (Foddy et al., 1990) and frog (Ozeki et al., 1991). More recently, large type lectins have been also isolated from a few mammals (Oda et al., 1993; Wada and Kanwar, 1997) and invertebrate (Kopác.k et al., 1993). Among the few amphibian species examined such as Rana pipiens (Roberson and Armstrong, 1980), Rana catesbiana (Ozeki et al., 1991; Uchiyama et al., 1997), Xenopus laevis (Marschal et al., 1992) and Bufo arenarum (Fink et al., 1987; Ahmed et al., 1996), a number of different lectin activities have been identified in various adult tissues, including skin, muscle and gonad. In the skin of Xenopus laevis, Marschal et al. (1992) demonstrated the presence of a b-galactoside-binding lectin in the granular glands around keratinocytes and proposed that skin lectins might mediate mechanisms associated with host defense.
In the toad Bufo arenarum Elola et al. (1998) determined the presence of a b-galactoside-binding lectin of 14.5 kDa in the ovary, oocytes and embryos, and suggested that it might play a role in embryo development.
The aim of this paper is the determination and characterization of b-galactoside-binding lectins in the skin of adult Bufo arenarum toads, with special emphasis on its possible antimicrobial activity.
Materials and Methods
Lectin purification
Sexually mature male Bufo arenarum specimens were collected during the breeding season (spring-summer) in the neighborhood of Salí river,Tucumán (Argentina). They were used immediately for experiments.
Bufo arenarum skin was dissected from ten adult males treated with 3% sodium pentobarbital and pooled. Then, 25 g of the tissue was homogeneized in a mortar for 30 min on ice with 50 ml of cold METBS buffer (50 mM Tris-HCl, pH=7.2; 150 mM NaCl; 10 mM EDTA and 4 mM mercaptoethanol) plus 150 mM lactose or 150 mM galactose Homogenates were centrifuged at 3,000 x g for 30 min. Supernatants were collected and centrifuged again at 100,000 x g for 1 h. These supernatants were extensively dialyzed against METBS to remove lactose and then lyophilized to produce a crude extract. All procedures were carried out at 4ºC.
A lactose binding lectin was purified from the crude extract, using a Sephadex G-100 gel filtration column (1.5 x 25.0 cm), equilibrated with METBS buffer, at 4ºC. The 13th to the 24th fractions (1.5 ml each) showed hemagglutination activity. These fractions were pooled and applied again to the same column. The resulting eluate fractions which showed hemagglutination activity were lyophilized and applied to an Agarose-lactose column (1 ml), equilibrated with METBS buffer, at 4ºC. The column was washed with the same buffer until the absorbance at 280 nm reached base line. The eluate obtained with METBS containing 0.3 M lactose was pooled, exhaustively dialyzed against METBS and lyophilized.
Determination of protein concentrations were carried out according to Lowry et al. (1951), with BSA as a protein standard.
Hemagglutination assays
Rabbit red blood cells were obtained by marginal vein puncture and various kinds of human red blood cells (types A, B and O) obtained from healthy volunteers were used. Rabbit erythrocytes were trypsin-treated and glutaraldehyde-fixed according to Roberson and Barondes (1982). Assays were performed using serial dilutions of the crude extract or with purified lectin in microtiter U-plates. Each well contained 25 ml of the lectin, 25 ml of TS (10 mM Tris-HCl, pH:7.6, plus 150 mM NaCl) with 1% bovine serum albumin (BSA) and 25 ml of 3% trypsin-treated glutaraldehyde-fixed rabbit erythrocytes. The mixtures were shaken and then hemagglutination activity was determined after 60 min at room temperature and scored from 0 (negative) to 4. Titer was defined as the reciprocal of the highest dilution giving a visible agglutination (Score of 1). Specific activity was defined as the ratio of titer to protein concentration (titer x mg/ml–1).
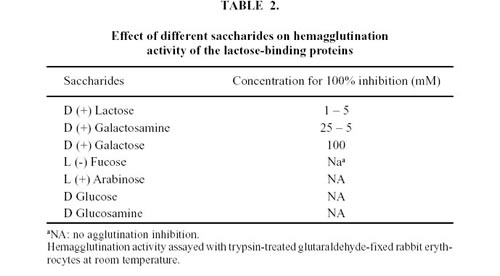
Hemagglutination inhibition assays were performed with different saccharides: D (+) Galactose, D (+) Galactosamine, D (+) Lactose, L (-) Fucose, L (+) Arabinose, D-Glucose and D-Glucosamine (Sigma, St. Louis, MO) at different concentrations. Inhibition of the lectin activity by different mono and oligosaccharides was carried out in microplates adding lectin and dilutions of each sugar. After incubation at room temperature for 30 min, 1% BSA and 3% suspension of trypsin treated glutaraldehyde-fixed rabbit erythrocytes were added. Plates were incubated for 1 h at room temperature. The concentration at which the saccharides exerted an inhibitory influence was estimated. The 100% lectin inhibition was defined as the lowest saccharide concentration that caused total inhibition of the hemagglutination activity under our working conditions.
A serial two-fold dilution series of the lactose-binding protein was made on a U-shaped multititer plate and subjected to an hemagglutination assay with 2 mM EDTA and 10 mM Ca++ for 1 h at room temperature.
In order to determine the effect of temperature, 100 ml samples were placed in Eppendorff and kept in a water bath for 30 min at temperatures ranging from 4°C to 100°C. After incubation the samples were centrifuged at 11,000 x rpm for 2 min and subsequently tested for hemagglutination activity as described above.
For the pH stability test, aliquots were dialyzed for 24 h both against Veronal: HCl buffer pH 3 - 9 and Glicine: Na(OH) buffer pH 10 - 12. After this period, dialysis in METBS buffer for 24 h was used to adjust the pH of the samples to its original value. Then, the samples were tested for hemagglutination activity.
Polyacrilamide gel electrophoresis
Affinity chromatography fractions were collected and analyzed by gel electrophoresis on 10% polyacrilamide gel in reducing and non-reducing conditions as described by Laemmli (1970). The gels were stained with silver reagent. Molecular weights were determined from standard curves with proteins of known molecular weights (Bio-Rad).
Preparative recovery of two bands of lactose-binding proteins from polyacrilamide geles were carried out using a electroeluter (Bio-Rad model 422).
Each protein band was cut from multiple gel slices and eluted with METBS buffer for 5 h at 4ºC. The eluted samples were collected and used for antimicrobial analysis.
Antimicrobial activity determination
Antibacterial activity of each protein band was assayed against Gram negative and Gram positive bacteria. A strain of Escherichia coli K12 4100 and a strain of wild Escherichia coli and Proteus morganii isolated from the skin of Bufo arenarum immediately captured of natural environment were tested. A strain of Enterococcus faecalis was assayed. Bacterial strains were maintained on different nutrient media (15 ml). All media were steam sterilized at 120°C for 20 min. To assay the inhibition of bacterial growth, the tested bacteria were homogeneously seeded with a sterile hissope on to Petri dishes containing 15 ml of the medium. Holes (7 mm in diameter) were aseptically bored into the agar with a hollow punch and were filled up with 25 ml of each lactose-binding protein at different concentrations (2 mg/ml; 1 mg/ml and 0.1 mg/ml). The plates were examined after incubation at 37ºC for 24 h. Bacterial growth inhibition was defined as the diameter was average of measurement per hole.
Experimental results between the different concentrations were analyzed using analysis of variance (ANOVA). Probability values < 0.05 were considered significant. Analyses were run in SPSS and Sigma Stat. Two-way analysis of variance was used to compare the sensibility of the different bacteria assayed.
To investigate whether the antibacterial action was bacteriostatic or bactericidal, growth inhibition was evaluated by plating bacteria from inhibition halo in the absence of lectin.
Results
Two saccharides, lactose (150 mM) and galactose (150 mM), were tested for their ability to extract lectin from Bufo arenarum skin. Both were found to be effective, with galactose showing a slightly lower ability than lactose.
When the crude extract was applied to a Sephadex G-100 column (2.0 x 25.0 cm), agglutination activity was detected from the 13th to the 24th fraction (Fig. 1). These fractions were pooled and concentrated to 1 ml, resulting in a 17-fold increase in specific activity (Table 1). Agglutination activity was optimum at pH 7.0-8.0. All further experiments were carried out at pH 7.2.
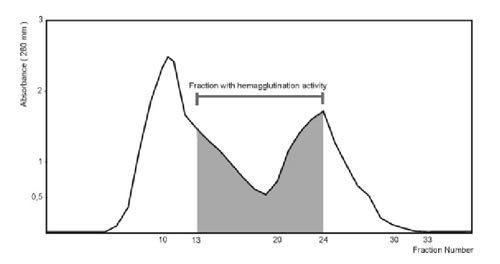
Figure 1. Purification of the lactose-binding proteins by Sephadex G-100 gel filtration.
Crude extract (4.265 mg) was applied to Sephadex G-100 gel filtration column. The column was equilibrated and washed with METBS buffer at 4°C. Fractions of 1.5 ml were collected and monitored by absorbance at 280 nm.
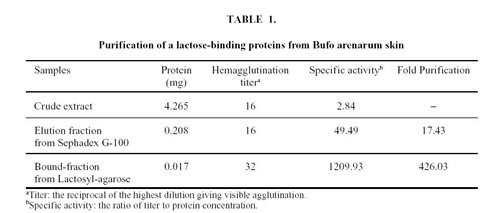
In order to purify lectin to homogeneity, the active fractions of Sephadex G-100 were loaded on a Lactosyl-agarose affinity chromatography and separated in two fractions: an unbound fraction that did not exhibit hemagglutination activity and a bound active fraction which was eluted with 300 mM lactose (Fig. 2). Total hemagglutination activities and specific activities for rabbit erythrocytes are presented in Table 1. The specific activity of the purified material was about 400 times higher than that of the crude extract.
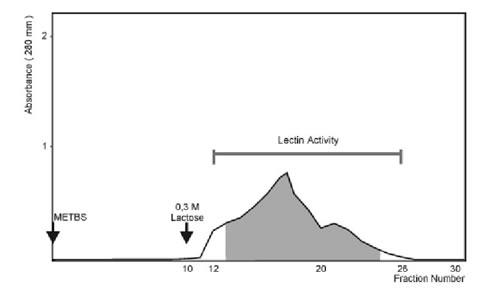
Figure 2. Purification of the lactose-binding proteins by Lactosyl-agarose affinity chromatography.
The eluate fractions to Sephadex G-100 gel filtration column, which showed hemagglutination activity, were applied to Agarose-lactose column (0.208 mg). The column was washed with METBS buffer at 4°C and eluted with the same buffer containing 0.3 M lactose (arrow).
The crude extract and the lactose-binding proteins strongly agglutinated trypsin-treated glutaraldehyde-fixed rabbit erythrocytes but failed to agglutinate human types O, A and B erythrocytes or trypsin-untreated rabbit erythrocytes.
The minimum concentration required for agglutination of the trypsin-treated rabbit erythrocytes was 0.65 µg/ml. The hemagglutination activity in METBS, pH 7.2, gradually decreased by repeated freezing and thawing, 5 cycles of freezing and thawing leading to a 95% loss of the initial hemagglutination activity. However, the lectin could be stored at 4ºC in the presence of excess lactose (0.5M) for at least three months with no loss of its hemagglutination activity. The toad skin lactose-binding proteins retained full agglutination activity when maintained at temperatures of approximately 30ºC. Activity decreased gradually between 42 and 60ºC and disappeared completely when the protein was heated at 75ºC for 30 min.
Removal of calcium from the assay by the addition of 2 mM EDTA had no effect on agglutination activity.
The carbohydrate-binding specificity of skin lectin was determined by analyzing the inhibition of the agglutination activity in the presence of several saccharides under optimal conditions.
The inhibition of the agglutination activity of the lactose-binding proteins are shown in Table 2. The most potent inhibitors were sugars bearing a b-D Galactoside configuration such as Lactose, D (+) Galactosamine and D (+) Galactose, in this order. The following sugars did not inhibit lectin activity when used at concentrations of up to 100 mM: D-Fucose, D-Arabinose, D-Glucose and D-Glucosamine. Similar results were obtained with LBP1 and LBP2 (data not shown).
The PAGE of Lactosyl-agarose affinity-isolated lectin revealed two bands under both reducing (Fig. 3) and non-reducing conditions (data not show), with a molecular weight 50 and 56 kDa respectively. The lectins were named LBP1 (50 KDa) and LBP2 (56 KDa)

Figure 3. A- PAGE of the lactose-binding proteins eluted from the Agarose-lactose column under reducing conditions.
B- PAGE of the unbound fractions from the Agarose-lactose column under reducing conditions.
At left Molecular mass markers: Phosphorylase b (97,400), Bovine Serum Albumin (66,200), Ovalbumin (45,000), Carbonic Anhydrase (31,000), Soybean Trypsin Inhibitor (21,500).
Animicrobial activity
To elucidate the antimicrobial activity of the lactose-binding proteins, we used a classical inhibition zone assay on thin agar. Results showed that both fractions obtained by electroelution, according to materials and methods, suppressed bacterial growth on solid medium showing activity against the Gram negative and Gram positive bacteria studied. As seen in Table 3, 25 mg of the fraction of 50 KDa (LBP1) showed different inhibition halos against the bacteria tested (Escherichia coli K12 strain 4100, Enterococcus faecalis and a strain of wild Escherichia coli and Proteus morganii isolated from toad skin). The other fraction of 56 KDa (LBP2) exhibited similar antibacterial activity. Proteus morganii being the most sensitive bacteria tested for LBP1 and LBP2 (Fig. 4). An ANOVA indicated a P value < 0.05 between experiments. The R.V.T. (Test of Scheffe) for every bacteria assay was around 55%, suggesting significant difference between each group (Fig. 5).
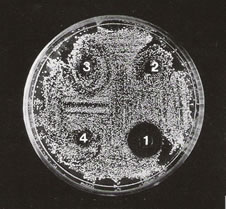
Figure 4. Growth inhibition of Proteus morganii with different lectin concentrations (LBP1): 1:25µg; 2: 2.5 µg; 3: 0.25 µg; 4: control METBS buffer. The medium used was Chocolate agar.

Figure 5. Median values of the grow inhibition at different lectin (LBP1) concentrations: a) Escherichia coli K 12 4100. b) Escherichia coli. c) Proteus morganii.

In our experimental conditions all bacteria tested grew again after removal from within the halo of inhibition, suggesting that both fractions were bacteriostatic.
Discussion
Several lectins have been purified from amphibians belonging to various species. They differ in their localization, solubility, molecular mass, number of units, cationic requirement and saccharide specificity. The amphibian skin synthetizes and releases an extraordinarily rich variety of biologically active substances (angiotensins, complex alkaloids, hormones and antibiotic peptides). In the present paper we detected b-galactoside-binding lectins from Bufo arenarum skin. Results suggest that the lectins purified from whole skin crude extract would be related to the group of S type lectins because it is soluble without detergents, is independent of the presence of calcium and interacts with lactose possibly because of its binding to the galactose residue, even though galactose by itself is a much poorer ligand than lactose.
The analysis of the optimal binding activity and stability of the skin lectin under a variety of experimental conditions revealed that these proteins can remain fully active for a long period of time if the intact proteins are stored in the presence of a soluble ligand, in this case lactose. This result agrees with the findings of Levi and Teichberg (1981) who, working with an electric eel lectin, reported that lactose protects the lectin against inactivation. Moreover, Lobsanov et al. (1993) suggested that both lactose and N- acetyl lactosamine used as a ligand maintain the lectin in its active conformation.
The pH range for the optimal binding activity of the skin lectins was rather narrow 7.0-8.0, the same as under natural physiological conditions. These results are similar to those obtained for the ovary galectin of the species by Ahmed et al. (1996). Like other lectins, the skin lectins were irreversibly inactivated by exposure to high temperatures for a relatively short period of time.
The electrophoretic behavior of the b-galactoside-binding proteins, both in native and in reducing conditions revealed the presence of two different proteins, LBP1 and LBP2, with molecular weights of 50 and 56 kDa respectively. Although these lectins seems to be different from the lactose-binding lectin of 16 kDa expressed in extreme abundance in Xenopus laevis skin (Marschal et al., 1992) where galectins are mainly confined. In a single species, different tissues may or may not express the some galectin. It was demonstrated in Bufo arenarum that a galectin of 14 KDa from embryos show inmunological cross reactivity with a galectin present in the ovary (Elola and Fink, 1996; Elola et al., 1998). Our results in the skin revealed differences with the 14 KDa galectin reported, they might be associated with substantially distinct biological functions, such developmental processes or host defense It would be another member of the S-Lac lectin family.
The presence of the lectins in the skin of Bufo arenarum led us to consider its possible biological involvement in the defense mechanisms of the species. The antimicrobial assays have shown that LBP1 and LBP2 presents strong activity against Gram negative bacilli such as Escherichia coli K12 strain 4100 and against species isolated from the skin of animals recently captured such as a wild strain of Escherichia coli and of Proteus morganii. Moreover, both fractions showed activity against Enterococcus faecalis. While antibiotic peptides present in the skin of other amphibian species have been known for years to have a strong antimicrobial activity (Zasloff, 1987), the Bufo arenarum lectins are the first lectins from frog reported to exhibit microbial growth inhibition. This activity was previously postulated for invertebrates (Jomori and Natori, 1992; Melo et al., 1998; Inamori et al., 1999), which expressed soluble and bound membrane lectin forms, that seemed to be one of the groups of molecules of recognition and defense. An instance of the above is provided by several types of hemocyte-derived lectins which may play a functional role in the innate immunity. The same idea was suggested for mammalian lectins, such as mannose-binding lectins of the collectin family, which play an important role in host-pathogen interactions by specific recognition with cell surface substances of bacteria (Sato and Hughes, 1994; Rabinovich et al., 1998). It is now well established that many of the bioactive substances present in the skin of amphibians have identical or closely related counterparts in mammals. Consequently, the results obtained from the former would be useful for comparative pharmacological and biochemical studies. The structural correspondence between amphibian and mammalian neuropeptides or antibiotic peptides have caused frog skin to be considered as a model for the study of new mammalian proteins, such as lectins.
In brief, the antibacterial activity of the b-galactoside binding lectins from Bufo arenarum skin is an event not yet reported in amphibians. We postulate that the innate inmmunity system of Bufo arenarum may recognize invading pathogens through a combined method using lectins with agglutinating and antimicrobial activities, and antibiotic peptides, not studied yet in this frog species, which would be released in response to injury. This fact might act synergistically to provide an effective host defense against invading microbes. Experiments are under way to determine the precise spectrum of antibiotic activity of the lactose-binding proteins, with especial emphasis on their action against fungi and Gram negative or Gram positive bacteria.
Acknowledgments
This research was supported by CIUNT grants to A. Sánchez Riera. We are gratefull to Dr. Raúl Salomón for providing the strain of Escherichia coli K12 4100. We wish to thank Mr. E. Dozetos for his photographically assistance, and Mrs. Virginia Méndez for her proofreading.
References
1. Ahmed H, Pohl J, Fink NE, Strobel F, Vasta GR (1996). The primary structure and carbohydrate specificity of a b-Galactosyl-binding lectin from toad (Bufo arenarum Hensel) ovary reveal closer similarities to the mammalian Galectin-1 than to the Galectin from the clawed frog Xenopus laevis. J Biol Chem 271, 51: 33083-33094. [ Links ]
2. Akimoto Y, Obinata A, Hirabayashi J, Sakakura Y, Endo H, Kasai K, Hirano H (1995). Changes in expression of two endogenous b-galactoside-binding isolectins in the dermis of chick embryonic skin during development in ovo and in vitro. Cell Tissue Res 279: 3-12. [ Links ]
3. Clark DP, Durell S, Malory WL, Zasloff M (1994). Ranalexin. A novel antimicrobial peptide from bull frog (Rana catesbiana) skin, structurally related to the bacterial antibiotic polymyxin. J Biol Chem 269: 10849-10855. [ Links ]
4. Elola M, Fink N (1996). Purification and partial biochemical characterization of an S-type lectin from blastula embryos of Bufo arenarum. Comp Biochem Physiol 115 B, 2: 175-182. [ Links ]
5. Elola M, Cabada M, Barisone G, Fink N (1998). Immunohistochemical localization of a galectin from Bufo arenarum ovary. Zygote 6: 1-9. [ Links ]
6. Erspamer V (1971). Biogenic amines and active polypeptides of the amphibian skin. Annu Rev Pharmacol 11: 327-350. [ Links ]
7. Erspamer V (1993). The opioid peptides of the amphibian skin. Int J Dev Neurosci 10: 3-30. [ Links ]
8. Fink de Cabutti NE, Caron M, Joubert R, Etola MT, Bladier D, Herkovitz J (1987). Purification and some characteristics of a b-Galactoside binding soluble lectin from amphibian ovary. FEBS Lett 223, 2: 330-334. [ Links ]
9. Foddy L, Stamatoglou SC, Hughes RC (1990). An endogenous carbohydrate-binding protein of baby hamster kidney (BHK21 C13). J Cell Sci 97: 139-148. [ Links ]
10. Inamori K, Saito T, Iwaki D, Nagira T, Iwanaga S, Arisaka F, Kawabata S (1999). A newly identified Horseshoe crab lectin with specificity for blood group A antigen recognizes specific O antigens of bacterial lipopolysaccharides. J Biol Chem 274, 6: 3272-3278. [ Links ]
11. Jomori T, Natori S (1992). Function of the lipopolysaccharides binding protein of Periplaneta americana as an opsonin. FEBS Lett 296: 283-286. [ Links ]
12. Joubert R, Caron M, Bladier D (1988). Distribution of b-Galactoside specific lectin activities during pre- and post-natal mouse brain development. Cell Mol Biol 34: 79-87. [ Links ]
13. Kopác.k P, Grubhoffer L, Söderhäll K (1993). Isolation and characterization of a haemagglutinin with affinity for lipopolysaccharide from the plasma of crayfish Pacifastacus leniusculus. Dev Comp Immunol 17: 407-418. [ Links ]
14. Kuchler K, Richter K, Trnarsky J, Egger R, Kreil G (1990). Two precursors of TRH from skin of X. Laevis: each contains seven copies of the end-product. J Biol Chem 265: 11731-11733. [ Links ]
15. Laemmli UK (1970). Cleavage of structural protein during assembly of the head of the bacteriophage T4. Nature 227: 680-685. [ Links ]
16. Levi G, Teichberg VI (1981). Isolation and physicochemical characterization of electrolectin, a ß-D-galactoside binding lectin from the electric organ of Electophorus electricus. J Biol Chem 256: 5735-5740. [ Links ]
17. Lobsanov YD, Gitt MA, Leffler H, Barondes SH, Rini JM (1993). X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9 resolution. J Biol Chem 268: 27034-27038. [ Links ]
18. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951). Proteins measurement with the Folin-phenol reagent. J Biol Chem 193: 265-275. [ Links ]
19. Marschal P, Herrmann J, Leffler H, Barondes SH, Cooper DNW (1992). Sequence and specificity of a soluble lactose-binding lectin from Xenopus laevis skin. J Biol Chem 267, 18: 12942-12949. [ Links ]
20. Melo VMM, Fonseca AM, Vasconcelos IM, Carvalho AFFU (1998). Toxic, antimicrobial and hemagglutinating activities of the purple fluid of the sea hare Aplysia dactylomela Rang, 1828. Braz J Med Biol Res 31, 6: 785-791. [ Links ]
21. Oda Y, Herrmann J, Gitt MA, Turck CW, Burlingame AL, Barondes SH, Leffler H (1993). Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J Biol Chem 268: 5929-5939. [ Links ]
22. Ozeki Y, Taei M, Kazuo N, Hiroaki K, Yoshio T, Koiti T (1991). Purification and characterization of b-Galactoside binding lectin from frog (Rana catesbiana) eggs. Biochem Biophys Res Commun 178, 1: 407-413. [ Links ]
23. Rabinovich GA, Iglesias MM, Modesti NM, Castagna LF, Wolfenstein-Todel C, Riera CM, Sotomayor CE (1998). Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T-cells: biochemical and functional characterization. J Immunol 160: 4831-4840. [ Links ]
24. Roberson MM, Armstrong PB (1980). Carbohydrate-binding component of amphibian embryo cell surface: restriction to surface regions capable of cell adhesion. Proc Natl Acad Sci USA 77: 3460-3463. [ Links ]
25. Roberson MM, Barondes SH (1982). Lectin from embryos and oocytes of Xenopus laevis. Purification and properties. J Biol Chem 257, 13: 7520-7524. [ Links ]
26. Sakakura Y, Hirabayashi Y, Oda Y, Ohyama Y, Kasai K (1990). Structure of chicken 16 kDa b-Galactoside-binding lectin. J Biol Chem 265: 21573-21579. [ Links ]
27. Sato S, Hughes RC (1994). Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem 269: 4424-4430. [ Links ]
28. Uchiyama H, Komazaki S, Oyama M, Matsui T, Ozeki Y (1997). Distribution and localization of galectin purified from Rana catesbiana oocytes. Glycobiology 7, 8: 1159-1165. [ Links ]
29. Wada J, Kanwar YS (1997). Identification and characterization of Galectin-9, a novel b-Galactoside-binding mammalian lectin. J Biol Chem 272, 9: 6078-6086. [ Links ]
30. Wells V, Mallucci L (1991). Identification of an autocrine negative growth factor: mouse b-Galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell 64: 91-97. [ Links ]
31. Zasloff M (1987). Magainins-antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84: 5449-5453. [ Links ]
Received on June 20, 2002.
Accepted on October 1, 2002.














