Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.27 n.1 Mendoza jan./abr. 2003
Inhibition of focal adhesion kinase by antisense oligonucleotides enhances the sensitivity of breast cancer cells to camptothecins
Taroh H. Satoh2, Tatiana A. Surmacz3, Okot Nyormoi4, and Cecilia M. Whitacre1
1. School of Natural and Health Sciences, Barry University, 11300 N.E. Second Ave., Miami Shores, FL 33161, USA.
2. Biochemistry and Molecular Genetics, University of Colorado Health Sciences Center, 4200 E. Ninth Ave., Denver, CO 80262, USA.
3. Max-Planck Research Unit Enzymology of Protein Folding, Weinbergweg 22, D-06120 Halle (Saale), Germany.
4. Department of Cancer Biology, University of Texas, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Address correspondence to: Cecilia Merched Whitacre, Ph.D. School of Natural and Health Sciences, Barry University, 11300 M.E. Second Avenue, Miami Shores, FL 33161-6695, USA. Fax: (+1-305) 899 3225. E-mail: cmerched@mail.barry.edu
Key words: focal adhesion kinase; drug resistance; camptothecins; breast cancer.
ABSTRACT: This study shows a strong association between cell attachment to substratum and activation of b1-integrin-signaling with resistance to the camptothecin derivative topotecan (TPT) in breast cancer cells. We propose a mechanistic-driven approach to sensitize the cells to camptothecins. ZR-75-1 anchorage-dependent breast cancer cell line, its derivative 9D3S suspension cells (9D3S-S), and 9D3S cells attached to fibronectin-coated plates (9D3S-A) were treated with TPT (1 mM) or CPT-11 (40 mM) for 48 h. Programmed cell death (PCD), as shown by poly(ADP-ribose) polymerase (PARP), pro-caspase-3 and pro-caspase-9 cleavage, was observed in 9D3S-S cells but not in ZR-75-1 or 9D3S-A cells. Because p125 focal adhesion kinase (FAK) is a transducer in the b1-integrin signaling pathway, it is essential to cell adhesion and it is overexpressed in metastatic breast cancer, we hypothesized that attenuation of FAK might enhance the sensitivity of breast cancer cells to camptothecins. Moreover, inhibition of FAK gene expression by a phosphorothioated antisense oligodeoxynucleotide targeting the portion of the gene encoding amino acids 262-268, increased the sensitivity of ZR-75-1, MDA-MB-231 and MCF7 breast cancer cells to treatment with TPT or CPT-11.
Introduction
Camptothecin, a pentacyclic alkaloid isolated from the Chinese tree Camptotheca acuminata (Hertzberg et al., 1989; Zhang et al., 1990), and its derivative topotecan (TPT) and the MDR 1-independent irinotecan (CPT-11) are important chemotherapeutic agents (Kingsbury et al., 1991; Rivory and Robert, 1995). Camptothecins bind to the topoisomerase (Topo) I-DNA complex during DNA replication, resulting in DNA damage (Rivory and Robert, 1995; Zhang et al., 1990) followed by programmed cell death (PCD), an important therapeutic indicator of their efficacy (Whitacre and Berger, 1997; Whitacre et al., 1999). A marker of PCD commonly used is the cleavage of the nuclear enzyme poly(ADP-ribose) polymerase (PARP) between Asp 216 and Gly 217. This proteolytic cleavage triggered by a cascade of caspases, splits the 116 kD enzyme into two fragments of about 90 kD and 26 kD (Whitacre and Berger, 1997; Whitacre et al., 1999). It has been observed that camptothecins appear to be more effective for the treatment of leukemia, with complete responses reported in patients and mouse model systems (Potmesil, 1994; Uckun et al., 1995), than for solid tumors. In concordance with these observations, the major toxicity described during treatment of solid tumors is hematologic (Kantarjian et al., 1993; Potmesil, 1994). Furthermore, leukemia cells are known to have a distinctive propensity to undergo PCD following treatment with camptothecins compared to the relatively lower response of colon cancer cell lines (Darzynkiewicz et al., 1996) and growth arrest rather than tumor regression in colon tumors (Rothenberg, 1996; Whitacre et al., 1999). Moreover, cell adhesion processes induce resistance to genotoxic agents (Fridman et al., 1990; Olive and Durand, 1994; St Croix and Kerbel, 1997; Uhm et al., 1999; Whitacre and Berger, 1997) and to radiation (Olive and Durand, 1994).
We have previously reported a strong association between cell adhesion to substratum and resistance to TPT in isogenic cervical cancer cell lines that differ in their attachment properties (Whitacre and Berger, 1997) as well as in a broad range of human cancer cell lines of various origins (Uhm et al., 1999; Whitacre and Berger, 1997). Focal adhesion kinase (FAK), a transducer in the b1-integrin signaling pathway reported to be essential to intercellular adhesion (Maung et al., 1999), has been suggested to also play an important role in cell survival (Frisch et al., 1996). Activation of integrin-signaling by binding to substratum or fibronectin results in phosphorylation of specific kinases including FAK, and activation of second messenger (Frisch et al., 1996; Hanks et al., 1992; Schaller et al., 1992). This activation of FAK subsequently suppresses induction of PCD (Frisch et al., 1996; Ruoslahti and Reed, 1994) in normal epithelial and endothelial cells. FAK is overexpressed in 88% of invasive and metastatic human breast tumors compared to normal tissue from the same individuals (Owens et al., 1995) and in several other types of cancer, including thyroid and lung, where it correlates with poor prognosis and aggressive phenotypes (Schaller et al., 1992). FAK and integrin-associated proteins also play an important role in proliferation and survival of prostate tumors and prostate cancer cell lines (Tremblay et al., 1996), and is therefore considered a marker of malignancy (Owens et al., 1995). Because of the high levels of FAK in solid tumors, and its function in cell adhesion and survival, we hypothesized that attenuation of FAK may sensitize cells to camptothecins. Moreover, it has been shown (Xu et al., 1998) that inhibition of FAK by targeting the portion of the gene encoding amino acids 262-268 by phosphorothioated antisense oligonucleotides induces cell detachment. This experimental approach has been used in this study to evaluate FAK as a potential target for anticancer therapy.
Materials and Methods
Cell lines
The ZR-75-1, MDA-MB-231 and MCF-7 breast cancer cell lines were originally obtained from the American Type Culture Collection (ATCC, Maryland, VA). The 9D3S cell line was derived by limiting dilutions from the ZR-75-1 cell line by Dr. Okot Nyormoi when he was in Dr. Michael A. Tainsky's laboratory at the University of Texas M.D. Anderson Cancer Center (Nyormoi et al., 2001). The ZR-75-1 and 9D3S cell lines were maintained in RPMI-1640 medium supplemented with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 10% heat inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA). MDA-MB-231 breast ductal carcinoma cells were kept in IMEM and MCF7 breast adenocarcinoma cells were cultured in MEM containing 10% FBS. Cells were maintained at 37°C in an atmosphere containing 5% CO2. Cells were observed under a conventional optical microscope (Hund Wetzlar Seiler W1352) and used during early logarithmic growth when both ZR-75-1 and its derivative 9D3S divided at a similar rate.
Western blot
Cells were lysed and sonicated in a solution comprised of 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate (SDS), 1% Triton X-100, 5 mM EDTA, 10 mg/ml aprotinin, 10 mg/ml leupeptin and 1 mM phenyl-methylsulfonyl fluoride (all reagents were from Sigma Chemical Co., St. Louis, MO). Samples containing 70 mg protein measured by Bio-Rad Dc protein assay (Bio-Rad Laboratories, Hercules, CA) were separated by SDS-PAGE consisting of a 5% (w/v) acrylamide stacking gel and a 12.5% (w/v) separating gel containing 0.1% SDS (5). The running buffer was comprised of 0.1% SDS, 25 mM Tris and 250 mM glycine (pH 8.3). Electrophoretic fractionation was carried out at a constant current of 15 mA. Proteins were then electrotransferred onto an Immobilon p15 membrane (Milipore Corp., Bedford MA). The filters were blocked with 5% nonfat dry milk in phosphate buffered saline FAK as potential anticancer target (PBS) containing 0.1% Tween-20 (PBS-Tween) for 5 min and then incubated overnight at 4°C with primary antibody at a concentration of 1 mg/ml in blocking solution. After washing in PBS-Tween, the filters were incubated overnight at 4°C in horseradish peroxidase-conjugated anti-immunoglobulin (1:1000). Following three washes in PBS-Tween, bands were visualized with enhanced chemiluminescence reagent and subsequent exposure to hyperfilm-enhanced chemiluminescence (Amersham Life Science Inc, Arlington Hights, IL). Intensity of the bands was quantified by densitometric scanning (SCIscan 5000 USB densitometer, United States Biochemical, Cleveland, OH) and normalized with respect to actin.
Source of antibodies
The monoclonal antibody to purified human PARP (Ranjit et al., 1995) is an IgG1k that shows specificity for the NAD-binding domain of PARP (PharMingen, San Diego, CA). Monoclonal anti-human pro-caspase-3 antibody was from Transduction Laboratories (Lexington, KY), and monoclonal antibody to pro-caspase-9 was from PharMingen. MAB2156 to FAK was from Chemicon International, Inc. (Tenecula, CA). Monoclonal antibody to actin and the horseradish peroxidase-linked anti-mouse immunoglobulins were from Amersham Life Science, Inc.
Acridine orange staining
An aliquot of 10 ml of a 100 mg/ml acridine orange (AO) stock solution prepared in PBS was added to 100 ml of single cell suspension. Immediately thereafter a minimum of 100 cells per point were counted under a fluorescence microscope. Cells with condensed chromatin in apoptotic bodies and cell membrane blebbing were counted as acridine orange positive whereas cells with homogenous chromatin were counted as acridine orange negative (Whitacre and Berger, 1997).
Antisense oligonucleotides
We have utilized phosphorothioated oligodeoxynucleotides to prevent their rapid degradation by ubiquitous nucleases. Since it has been previously shown that a 20-mer oligonucleotide targeted to the portion of the FAK gene encoding amino acids 262-268 (Xu et al., 1998) induces cell detachment from substrate we have selected this sequence for the present study. The oligonucletide was named AS2 (5'-ATAATCCAGCTTGAACCAAG-3'). A 2-base mismatched oligonucleotide named MS2-2 (5'-ATAATCGAGCTTCAACCAAG-3'), 5-base mismatched oligonucleotide named MS2-5 (5'-ATAATCGACGTTCAAGCAAG-3') and 6-base mismatched oligonucleotide named MS2-6 (5'-ATAAGCGACGTTCAA GCAAG-3'), were used as negative controls. N-terminal fluorescein isothiocyanate (FITC)-labeled oligodeoxynucleotides were used to assess cellular uptake. The oligonucleotides were from (MWG-Biotech Inc, Highpoint, NC).
Antisense treatment
ZR-75-1, MDA-MB-231 and MCF7 cells were plated in 3 ml of their respective culture medium at a density of 105 cells per well in 6-well plates and incubated at 37°C in an atmosphere of 5% CO2. Following 24 h incubation the culture medium was replaced with serum-free Opti-MEM (Life Technology Inc., Carlsbad, CA). Cells were then transfected with 0.1-10 mM AS2 or one of the MS2 oligonucleotides with 10 mg/ml lipofectamine (LPA) reagent (Life Technology Inc.) following the manufacturer's instructions. Time-course and dose-response for inhibition of FAK expression were assessed by Western blot.
Treatment with camptothecins
After pretreatment of cells with LPA alone, AS2 or MS2 in serum-free Opti-MEM, as described above, heat inactivated FBS was added to a final concentration of 10%. Stock solutions of TPT and CPT-11 were prepared in dimethyl sulfoxide (DMSO) and diluted 1:1000 in the culture media to final concentrations of 0.1-5 mM TPT or 10-40 mM CPT-11. Cells were then incubated for an additional 24-48 h at 37°C. Lysates were prepared from treated cells containing both adherent and non-adherent fractions, to assess PARP cleavage by Western blot.
Results
Cell adhesion and resistance to camptothecins
To demonstrate the relationship between cell adhesion and resistance to the pentacyclic alkaloids camptothecins (Fig. 1), we examined the ZR-75-1 and its derivative 9D3S breast cancer cell lines. Intercellular adhesion of ZR-75-1 cells is strong, and the cells anchor to polystyrene flasks, whereas 9D3S cells grow as a single cell suspension (9D3S-S) (Fig. 2A). 9D3S cells were induced to attach (9D3S-A) to 6-well plates coated with 40 mg fibronectin per well (Fig. 2) to evaluate the effect of activation of b1-integrin signaling pathway on the sensitivity of the cells to TPT or CPT-11. ZR-75-1, 9D3S-A and 9D3S-S cells, all in logarithmic growth and similar doubling time, were treated with increasing concentrations of TPT (0.1-1 mM) or CPT-11 (10-40 mM) for various times (5 min-72 h). The percentage of cells in PCD as assessed by AO staining was much greater for 9D3S-S cells than for 9D3S-A or ZR-75-1 cultures. Figure 2B shows the results obtained after 48 h treatment with 0.1 mM TPT or 40 mM CPT-11, conditions that enabled to visualize the most clear differential pattern of sensitivity between adherent and suspension cells. About 21% of 9D3S-S cells showed chromatin condensation following treatment with 1 mM TPT for 48 h, whereas only ~2-5% of the ZR-75-1 or 9D3S-A cells were AO positive under the same experimental conditions (Fig. 2B). Likewise, treatment with 40 mM CPT-11 for 48 h resulted in ~11% AO positive of 9D3S-S cells, whereas only about 3-4% of 9D3S-A or ZR-75-1 cells were AO positive (Fig. 2B). Furthermore, the low percentage of TB positive cells with a parallel pattern to the AO response suggested that necrosis probably occurred secondary to PCD (Fig. 2B). PARP cleavage (Fig. 2CD) and degradation of pro-caspase-3 and pro-caspase-9 (Fig. 2C) were assessed as indicators of PCD. In agreement with the results shown with acridine orange staining, 9D3S-S cells showed markedly higher levels of PARP cleavage and degradation of pro-caspase-3 and pro-caspase-9 compared with the ZR-75-1 (Fig. 2C) or the 9D3S-A cells (Fig. 2D) after treatment with 1 mM TPT or 40 mM CPT-11 for 48 h. Actin was used as internal standard for protein loading. Our results suggest a strong association between breast cancer cell adhesion and resistance to TPT or CPT-11.
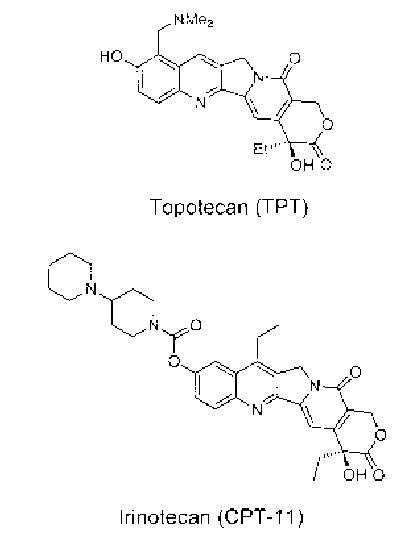
Figure 1. Chemical structure of TPT and CPT-11.

Fig. 2A
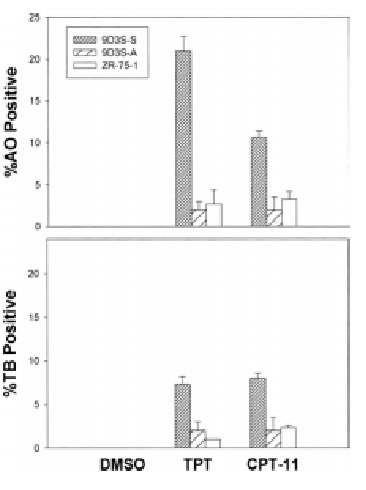
Fig. 2B

Fig. 2C
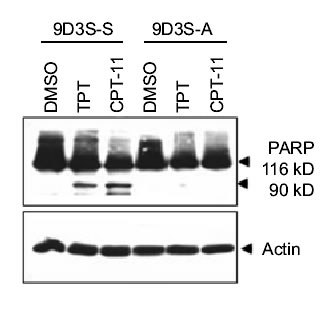
Fig. 2D
Figure 2. Association between cell adhesion status and resistance to camptothecins. (A). ZR-75-1 breast adenocarcinoma cells anchored to polystyrene flasks, 9D3S-A cells attached to fibronectin-coated plates and 9D3S-S suspension cell. (B). Treatment with 1 mM TPT or 40 mM CPT-11 for 48 h induced chromatin condensation and apoptotic acridine orange staining in 9D3S-S cells. Necrosis was assessed by trypan blue uptake. The results are the mean value of triplicates + standard deviation. (C). Western blot of ZR-75-1 and 9D3S-S cells treated with 1 mM TPT or 40 mM CPT-11 for 48 h. TPT and CPT-11 induced PARP cleavage and degradation of pro-caspase-9 and pro-caspase-3 in 9D3S-S cells at 48 h, whereas this response was markedly delayed in ZR-75-1 and (D) PARP cleavage was delayed in 9D3S-A cells compared with 9D3S-S cells. Actin was used as internal standard for protein loading.
Attenuation of FAK by antisense oligonucleotides
The antisense oligonucleotides used in this study specifically inhibit the translation of encoded proteins by hybridizing to the target mRNA through Watson-Crick complementary base recognition. To evaluate the effect of attenuation of FAK on the sensitivity of cells to camptothecins we have selected three breast cancer cell lines expressing FAK (Fig. 3B): ZR-75-1, MDA-MB-231 and MCF7 cells. In order to assess uptake of the oligonucleotides, cells were transfected with FITC-labeled antisense as described in Materials and Methods. Fluorescence was observed within the cells under an inverted fluorescence microscope about 4 h after transfection and remained visible approximately for 24 h. Background fluorescence was absent from untreated cells (data not shown). We have evaluated various doses (0.1-10 mM) and times (24-72 h) of treatment with the antisense oligonucleotides and found optimal inhibition of FAK at 3 mM AS2 for 24 h. Figure 3A shows positive uptake of N-terminal FITC-labeled AS2 or MS2 (3 mM) by ZR-75-1, MDA-MB-231 and MCF7 cells following 24 h incubation. Western blotting against FAK showed decrease of protein levels in the AS2 treated group but not in cells treated with LPA alone or the MS2 mismatched oligonucleotides (Fig. 3B). Since all three MS2 oligonucleotides analyzed (MS2-2, MS2-5 and MS2-6) resulted in identical responses, only the results obtained with MS2-2 are shown. The degree of attenuation of FAK varied from one experiment to another with apparently greater efficacy in the treatment of the MDA-MB-231 cells compared with the ZR-75-1 cells, despite the fact that both cell lines express similar high endogenous levels of the target protein. We suggest that this effect may be related to differences in the efficiency of transfection shown in Figure 3A with FITC-labeled oligonucleotides. AS2 did not affect the ability of the cells to produce colonies (data not shown) and showed little (<5% PARP cleavage) cytotoxicity (Fig. 5AB). Some cells treated with AS2 detached from the polystyrene flasks after gentle rotation whereas MS2 and LPA treated cells remained attached (Fig. 4).
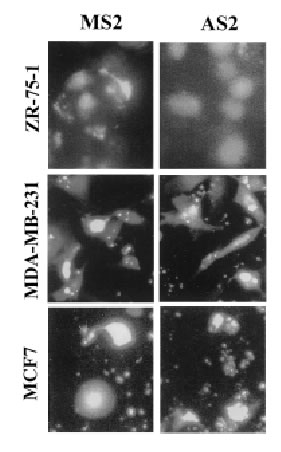
3A
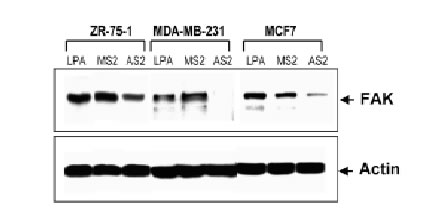
3B
Figure 3. Effect of AS2 on FAK expression. (A). ZR-75-1, MDA-MB-231 and MCF7 cells in logarithmic growth were treated with 3 mM FITC-labeled AS2 or MS2 for 24 h. MDA-MB-231 and MCF7 cells show higher uptake of oligonucleotides than ZR-75-1 cells. (B). AS2 markedly reduced endogenous levels of FAK as measured by Western blotting. Actin was used as internal standard for protein loading. LPA and a two-base mismatched oligonucleotide (MS2) were used as negative controls.

Figure 4. Effect of AS2 on cell adhesion. ZR-75-1, MDA-MB-231 and MCF7 cells in logarithmic growth were treated with 3 mM AS2 or MS2 for 24 h. AS2 transfected cells detached after rotation of the plates more readily than the MS2 or LPA-transfected control cells.
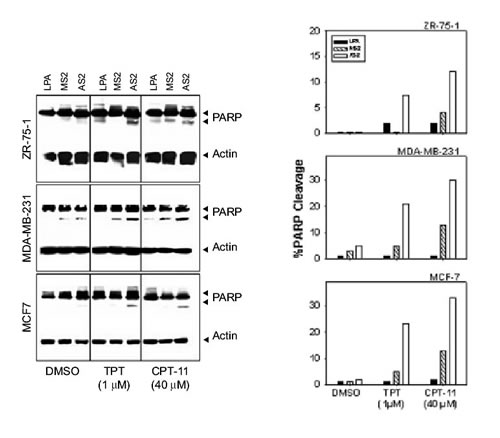
Figure 5. Attenuation of FAK sensitizes breast cancer cells to camptothecins. (A) Cells in logarithmic growth were treated with 3 mM AS2 for 24 h as described in Materials and Methods. Cells treated with LPA or MS2 were used as negative controls. Subsequently, 1 mM TPT or 40 mM CPT-11 was added onto the cells. Combination treatment with AS2 and TPT or CPT-11 showed greater toxicity, as assessed by PARP cleavage. The 116 and 90 kD bands correspond to reactivity with PARP antibody, whereas the 46 kD band corresponds to actin used as internal standard for protein loading. (B) Densitometric profile of the results shown in A.
AS2 enhances the sensitivity of breast cancer cells to camptothecins
Twenty-four h after transfection with LPA alone, MS2 or AS2 (3 mM), cells were treated with increasing doses of TPT or CPT-11 for 24 h (0.1-10 mM TPT or 10-40 mM CPT-11). Cells were then harvested and PARP cleavage was examined by Western blot. Optimal results were observed with 1 mM TPT and 40 mM CPT-11. Figure 5 shows ~8-24% PARP cleavage after treatment with 1 mM TPT for 24 h and 13-33% increase in PARP cleavage after treatment with 40 mM CPT-11 for 24 h in AS2 treated cells compared with control cells treated only with TPT (2-3% PARP cleavage), CPT-11 (2-4% PARP cleavage) or AS2 (1-5% PARP cleavage). Control cells treated with LPA or MS2 alone also showed trace levels of PARP cleavage. Furthermore, treatment with AS2 followed by CPT-11 induced about 2-3 times the levels of PARP cleavage compared to the corresponding values for the MS2/CPT-11 treated cells. Similarly, AS2 pretreatment increased the sensitivity of the cells to TPT at least 3 fold as compared to the level observed for the MS2 pretreated cells (Fig. 5).
Discussion
It is well established that most solid tumors are intrinsically resistant to chemotherapy. Furthermore, after chemotherapeutic treatments the residual tumor cells become increasingly resistant (Glinsky, 1998). Overcoming this drug resistance phenotype in solid tumors is a major challenge during drug development. We have previously observed that interference with integrin-signaling sensitizes cervical cancer cells to treatment with TPT (Whitacre and Berger, 1997). Since the strong correlation between the percentage of PCD measured by the PARP cleavage assay and by acridine orange staining is well recognized (Whitacre and Berger, 1997), we have utilized both methods to assess PCD in this study. Here we further demonstrate an association between cell anchorage status and sensitivity to TPT or CPT-11 in isogenic human breast cancer cell lines. Our results show that adherent cells (ZR-75-1 and 9D3S-A) are more resistant to TPT or CPT-11 than suspension cells (9D3S-S). Because FAK is overexpressed in metastatic breast cancer and in breast cancer cell-lines and is a transducer in the b1-integrin signaling pathway, we have hypothesized a potential role for this protein in the resistance phenotype to camptothecins. To directly test the relationship between cell attachment and sensitivity to Topo I inhibitors we have treated the anchorage-dependent ZR-75-1, MDA-MB-231 and MCF7 breast cancer cells with a phosphorothioated antisense oligonucleotide against FAK previously shown to induce melanoma cell detachment. In this study we demonstrate that attenuation of FAK sensitizes breast cancer cells to the camptothecin analogs TPT and CPT-11. Several pro-survival mechanisms have been suggested for FAK including a regulatory role on the conformation of the Bax BH3 epitope (Gilmore et al., 2000), with consequences on p53-mediated PCD (Ilic et al., 1998). FAK is known (Sonoda et al., 2000) to promote activation of the anti-apoptotic factor NFkB by phosphorylation of IkBa. In addition, it is possible that changes in cell adhesion status may impact cell cycle progression, therefore affecting the sensitivity of cells to subsequent treatment with Topo I inhibitors. Because FAK is required for tumor cell adhesion (Maung et al., 1999), the use of antisense to FAK along with TPT or CPT-11 may be useful for in vivo studies in animals. FAK is the center of an intricate mesh of signaling pathways controlling cell proliferation, adhesion, survival and migration, all related to tumor growth, suggesting that attenuation of FAK may also affect these pathways in vivo. There is the concern that interference with cell adhesion in vivo may favor metastasis. Nonetheless, since FAK is also involved in angiogenesis, attenuation of FAK could also impair cancer progression. Hence, our results point to FAK as potential target for the development of novel anticancer treatments.
Acknowledgements
This study was supported by the National Cancer Institute (KO1 CA77065 grant). We would like to thank Mariela Roldan-Olarte and Edith I. Yslas for their cooperation and Dr. Pablo A. Valdecantos for the critical reading of the manuscript.
References
1. Darzynkiewicz Z, Bruno S, Del Bino G, Traganos F (1996). The cell cycle effects of camptothecin. Ann N Y Acad Sci 803: 93-100. [ Links ]
2. Fridman R, Giaccone G, Kanemoto T, Martin GR, Gazdar AF, Mulshine JL (1990). Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci USA87: 6698-6702. [ Links ]
3. Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY (1996). Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 134: 793-799. [ Links ]
4. Gilmore AP, Metcalfe AD, Romer LH, Streuli CH (2000). Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol 149: 431-446. [ Links ]
5. Glinsky GV (1998). Anti-adhesion cancer therapy. Cancer Metastasis Rev17: 177-185. [ Links ]
6. Hanks SK, Calalb MB, Harper MC, Patel SK (1992). Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA 89: 8487-8491. [ Links ]
7. Hertzberg RP, Caranfa MJ, Hecht SM (1989). On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 28: 4629-4638. [ Links ]
8. Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH (1998). Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol 143: 547-560. [ Links ]
9. Kantarjian HM, Beran M, Ellis A, Zwelling L, O'Brien S, Cazenave L, Koller C, Rios MB, Plunkett W, Keating MJ, and et al. (1993). Phase I study of Topotecan, a new topoisomerase I inhibitor, in patients with refractory or relapsed acute leukemia. Blood 81: 1146-1151. [ Links ]
10. Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK, and et al. (1991). Synthesis of water-soluble (aminoalkyl)camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem 34: 98-107. [ Links ]
11. Maung K, Easty DJ, Hill SP, Bennett DC (1999). Requirement for focal adhesion kinase in tumor cell adhesion. Oncogene 18: 6824-6828. [ Links ]
12. Nyormoi O, Wang Z, Doan D, Ruiz M, McConkey D, Bar-Eli M (2001). Transcription factor AP-2alpha is preferentially cleaved by caspase 6 and degraded by proteasome during tumor necrosis factor alpha-induced apoptosis in breast cancer cells. Mol Cell Biol 21: 4856-4867. [ Links ]
13. Olive PL, Durand RE (1994). Drug and radiation resistance in spheroids: cell contact and kinetics. Cancer Metastasis Rev13: 121-138. [ Links ]
14. Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG (1995). Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 55: 2752-2755. [ Links ]
15. Potmesil M (1994). Camptothecins: from bench research to hospital wards. Cancer Res 54: 1431-1439. [ Links ]
16. Ranjit GB, Cheng MF, Mackay W, Whitacre CM, Berger JS, Berger NA (1995). Poly(adenosine diphosphoribose) polymerase in peripheral blood leukocytes from normal donors and patients with malignancies. Clin Cancer Res 1: 223-234. [ Links ]
17. Rivory LP, Robert J (1995). Molecular, cellular, and clinical aspects of the pharmacology of 20(S)camptothecin and its derivatives. Pharmacol Ther 68: 269-296. [ Links ]
18. Rothenberg ML (1996). The current status of irinotecan (CPT-11) in the United States. Ann N Y Acad Sci 803: 272-281. [ Links ]
18. Ruoslahti E, Reed JC (1994). Anchorage dependence, integrins, and apoptosis. Cell 77: 477-478. [ Links ]
19. Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT (1992). pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA89: 5192-5196. [ Links ]
20. Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T (2000). Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem275: 16309-16315. [ Links ]
21. St Croix B, Kerbel RS (1997). Cell adhesion and drug resistance in cancer. Curr Opin Oncol 9: 549-556. [ Links ]
22. Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S (1996). Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer 68: 164-171. [ Links ]
23. Uckun FM, Stewart CF, Reaman G, Chelstrom LM, Jin J, Chandan-Langlie M, Waddick KG, White J, Evans WE (1995). In vitro and in vivo activity of topotecan against human B-lineage acute lymphoblastic leukemia cells. Blood 85: 2817-2828. [ Links ]
24. Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL (1999). Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res 5: 1587-1594. [ Links ]
25. Whitacre CM, Berger NA (1997). Factors affecting topotecan-induced programmed cell death: adhesion protects cells from apoptosis and impairs cleavage of poly(ADP- ribose)polymerase. Cancer Res57: 2157-2163. [ Links ]
26. Whitacre CM, Zborowska E, Willson JK, Berger NA (1999). Detection of poly(ADP-ribose) polymerase cleavage in response to treatment with topoisomerase I inhibitors: a potential surrogate end point to assess treatment effectiveness. Clin Cancer Res5: 665-672. [ Links ]
27. Xu LH, Yang X, Craven RJ, Cance WG (1998). The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ 9: 999-1005. [ Links ]
28. Zhang H, D'Arpa P, Liu LF (1990). A model for tumor cell killing by topoisomerase poisons. Cancer Cells 2: 23-27. [ Links ]
Received on November 21, 2002.
Accepted on February 19, 2003.














