Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.27 no.1 Mendoza Jan./Apr. 2003
Micropropagation of Glandularia perakii Cov. et Schn. (Verbenaceae), a native species with ornamental potential
Concepción Marino1, María T. Ponce1*, María E. Videla2, Sonia Fioretti3 and Miguel Cirrincione1.
1. Fisiología Vegetal. Dpto Ciencias Biológicas. Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
2. Botánica Agrícola. Dpto Ciencias Biológicas. Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
3. Espacios Verdes. Dpto Producción Agropecuaria. Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo.
Address correspondence to: Ing. Agr. María Teresa Ponce. Cátedra de Fisiología Vegetal. Dpto. Ciencias Biológicas, Facultad de Ciencias Agrarias, Universidad Nacional de Cuyo. Alte. Brown 500, (5505) Chacras de Coria, Mendoza, ARGENTINA. E-mail: mponce@fca.uncu.edu.ar
Keywords: tissue culture, in vitro propagation, nodal segment, Glandularia perakii.
ABSTRACT: Glandularia perakii is a perennial species with beautiful violet flowers that grows in the stony soil of Mendocine pedemont. A plentiful and prolonged flowering confers it an important ornamental potential. In this paper, a method of propagation of G. perakii from nodal segments is reported. Proliferating microshoot cultures were obtained by placing nodal segment on Murashige and Skoog medium (MS) supplemented with 20 g.L-1 of sucrose without growth regulators. In this medium multiplication rate after 20 days was 7.9. Rooted plants were acclimatized successfully.
Abbreviations: MS Murashige and Skoog medium; BA _ 6-benzyladenine; IBA _ indole-3-butyric acid.
Introduction
Indigenous herbaceous plants have a limited use as ornamentals in gardens and parks in Argentina, mainly due to the lack of knowledge about cultivation conditions (Cesare et al., 1997).
Glandularia perakii Cov. et Schn. (Verbenaceae) is an interesting native species from the arid pedemont (Troncoso, 1974). It grows in stony soils in the Precordillera area of Mendoza and San Juan in Argentina. It is a perennial species, with erect or suberect, prostate stem of 40-60 cm long, triparted-pinnatelobed leaves, and flowers with a violet corolla clustered in solitary and terminal spikes (Covas and Schnack, 1944).
The plant habit, showy flowers and the abundant and prolonged flowering season justify the introduction to cultivation of this native species.
No satisfactory results were obtained in propagation of G. perakii from seeds or herbaceous cuttings (Videla et al., 2001).
The aim of the present work was to develop a micropropagation method for G. perakii from nodal segments in order to produce abundant material to evaluate its ornamental potential.
Materials and Methods
Plants of G. perakii obtained from Papagallos basin (32° 12' S, 32° 12' W), department of Las Heras, Mendoza Province, Argentina were grown in a greenhouse for 20 days. Single nodes, 1-2 cm long, were taken from elongating young shoots to initiate the culture. Explants were thoroughly washed with water and surface sterilized by immersion in ethanol 70% for 1 min and in 15% commercial bleach (0.8% sodium hypochlorite, final concentration) for 10 min, finally washed three times with sterile water.
Nodal segments were implanted vertically in 20 mm x 15 mm glass tubes containing 20 ml basal MS medium, (Murashige Skoog, 1962), supplemented with 20 g.l-1 sucrose and agar (0.8% w/v). The pH of all media was adjusted to 6 before adding agar and then autoclaved for 15 min at 121ºC.
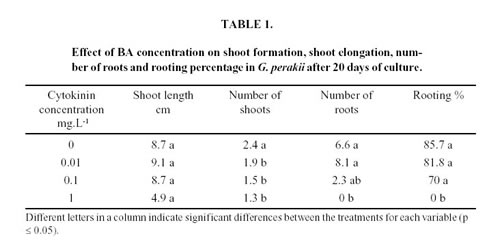
At the establishment stage, different BA concentrations were tested: 0; 0.01; 0.1 and 1 mg.L-1. Twenty-five explants were cultured for each treatment. Twenty days after incubation, infection percentage, survival percentage, hyperhydric plants percentage, number and length of shoots (only shoots larger than 0.5 mm newly differentiated from the explants), number of roots and rooting percentage were determined.
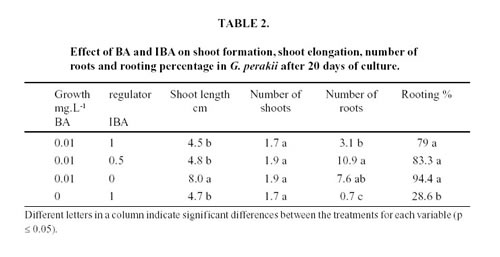
First subculture: microplants derived from explants established in vitro were cut into 0.5 - 1 cm long nodal segments and placed in 20 mm x 15 mm glass tubes containing the same basal medium with the following growth regulators 0,01 mg.L-1 BA + 1 mg.L-1 IBA; 0,01 mg.L-1 BA + 0.5 mg.L-1 IBA; 0,01 mg.L-1 BA; and 1 mg.L-1 IBA. Twenty explants for each treatment were cultured. Twenty days after incubation, number and length of shoots, number of roots and rooting percentage were determined.
Second subculture: microplants from the first subculture were used to obtain 0.5-1 cm long nodal segments, they were placed in 360 ml glass flasks (6 segments in each) with 60 ml of the same basal medium with 20 or 30 g.L-1 of sucrose and with or without 0,01 mg.L-1 of BA. Fifteen flasks for each treatment were cultured. Twenty days later, number and length of shoots, number of nodes, number of roots, rooting percentage and dry weight were determined.
All cultures were maintained at 25º ± 2ºC with 16-h photoperiod using fluorescent lamps (100 mmol.m-2.s-1 photosynthetically active radiation).
Microplants from the second subculture were washed in tap water and transferred to plastic pots containing a sterilized mixture of exhausted grape bagasse and sand (8:2) and then kept in a growth chamber, which was gradually opened during the acclimation period of 20 days. In acclimatized plants, number of shoots, number of nodes, leaf area and dry weight were determined.
The experimental design in all experiments was fully randomized. Data were subjected to analysis of variance and differences between means were tested using Tukey test. Percentages were analyzed by arcsine test according to Capelletti (1982).
Results and Discussion
At the establishment stage, approximately 65% of explants were free from contamination. No significant differences between treatments were found in survival percentage (Fig. 1).
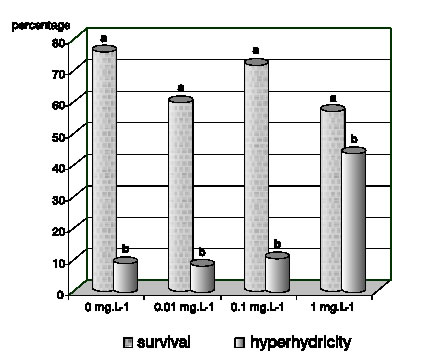
Figure 1. Effect of BA concentration on survival percentage and hyperhydricity percentage in G. perakii explants after 20 days of in vitro culture. Different letters on the column indicate significant differences between the treatments for each variable (p £ 0.05).
Hyperhydricity was observed in all treatments, but shoots cultured in the medium with the highest BA concentration showed the highest percentage of hyperhydric plants (Fig. 1). Although several factors are generally involved in the induction or development of shoot hyperhydricity, an excess of cytokinins has been reported to be a major factor involved in shoot hyperhydricity during in vitro vegetative propagation of several plant species, (Kataeva et al., 1991). This fact may explain the obtained results. Different concentration of BA added to the medium did not statistically affect shoot length, although there was a significant decrease in shoot number with this growth regulator. The number of roots was lower than the control with 0.1 mg.L-1 of BA and was inhibited when this concentration was 1 mg.L-1. The highest concentration of BA also inhibited the formation of roots (Table 1). Sauvaire and Galzy (1981) had already described this inhibitory effect of cytokinins on rooting in sugar cane.
In the first subculture the best shoots growth was obtained with 0.01 mg.L-1 of BA but no differences between treatments were found in the number of shoots. The medium supplemented with 0.01 mg.L-1 of BA in combination with 0.5 mg.L-1 of IBA was the most effective in terms of root production (Table 2).
In the second subculture, no significant differences were found between treatment in shoot length, number of nodes, number of roots, dry weight and rooting percentage (data not shown). The shoots grew between 7.4 and 11.5 cm and the rooting percentage was higher than 90 %. The average multiplication rate was 7.9. Similar results were observed in Lippia integrifolia (Passera and Ambrosetti, 1999).
Acclimatization, the transition from in vitro to ex vitro conditions, is the final step in any micropropagation scheme. Increasing the carbohydrate level in the culture medium before in vitro transfer may improve the quality of established plants (Wainwright and Scrace, 1989). Nevertheless, G. perakii showed to be insensitive to increasing sucrose concentration from 20 to 30 g.L-1 since survival percentage, number of shoots, number of leaves, leaf area, and dry weight did not show significant difference between treatments at the acclimatization stage (data not shown).
The plants propagated by tissue culture did not show any visual morphological abnormality when compared with the original plants.
By using solid MS medium supplemented with 20 g.L-1 of sucrose without growth regulators, satisfactory regeneration yield of abundant plants can be obtained within a short period.
Acknowledgments
This study was financially supported by the Consejo de Investigaciones de la Secretaría de Ciencia y Técnica de la Universidad Nacional de Cuyo. Project number 06/A138.
The authors thanks Ing. Olga Fernandez and Dr. Carlos Passera for helpful suggestions on the manuscript.
References
1. Capelletti CA (1982). Elementos de estadística. Eds. Cesarino Hnos., Madrid, Chap. 10, p 233-254. [ Links ]
2. Cesare SM, Meeham AR, Boetto MN (1997). Plantas Nativas. Su uso en espacios verdes urbanos. Eds Educor, Córdoba. p 9. [ Links ]
3. Covas G, Schnack B (1944). Tres nuevas especies de Glandularia de la flora argentina. Revista Argentina de Agronomía. 11(2):89-97. [ Links ]
4. Kataeva NV, Alexandrova IG, Butenko RG, Dragavtceva EV (1991). Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tiss. Org. Cult. 27: 149-154. [ Links ]
5. Murashige T, Skoog FM (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473-497. [ Links ]
6. Passera CB, Ambrosetti JA (1999). In vitro propagation of "Incayuyo", Lippia integrifolia (Gris.) Hier. (Verbenaceae), a medicinal and aromatic plant of Monte phytogeographical province, Argentina. Acta Hort. 502: 319-323. [ Links ]
7. Sauvaire D, Galzy R (1981). Micropropagation de la canne a sucre par bouturage in vitro Action d'une auxine et d' une cytokinine. L'Agronomie Tropicale (1):65-68. [ Links ]
8. Troncoso NS (1974). Los géneros de Verbenáceas de Sudamérica extratropical. Darwiniana 18 (3-4): 295-412. [ Links ]
9. Videla ME, Fioretti S, Marino C (2001). Introducción al cultivo de especies nativas: Glandularia perakii. III Jornadas Nacionales de Floricultura. Noviembre. Mendoza. Argentina. Publicado en CD. [ Links ]
10. Wainwright H, Scrace J (1989). Influence of in vitro preaconditioning with carbohydrates during the rooting of microcuttings on in vivo establishment. Scientia Horticulturae, (38): 261-267. [ Links ]
Received on April 15, 2002.
Accepted on September 13, 2002.














