Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.27 n.2 Mendoza abr./ago. 2003
Role of mast cells in gastrointestinal mucosal defense
Alicia B. Penissi1, María I. Rudolph2, Ramón S. Piezzi1
1 Instituto de Histología y Embriología "Dr. Mario H. Burgos" (IHEM-CONICET), Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Casilla de Correo 56, (5500) Mendoza, Argentina.
2 Departamento de Farmacología, Facultad de Ciencias Biológicas, Universidad de Concepción, Casilla 160-C, Concepción, Chile.
Address correspondence to: Alicia B. Penissi, PhD. Instituto de Histología y Embriología «Dr. Mario H. Burgos», Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Casilla de Correo 56, (5500) Mendoza, ARGENTINA. Fax: (+54-261) 449 4117; E-mail: apenissi@fcm.uncu.edu.ar
Abstract: The purpose of this review, based on studies from our laboratory as well as from others, is to summarize salient features of mast cell immunobiology and to describe their associations with gastrointestinal mucosal defense. Gastrointestinal mast cells are involved in many pathologic effects, such as food hypersensitivity. On the other hand, they also play a protective role in defense against parasitic and microbial infections. Thus, they have both positive and negative effects, but presently the mechanisms that control the balance of these various effects are poorly known. It has been suggested that stabilization of mast cells may be a key mechanism to protect the gastrointestinal tract from injury. Few molecules are known to possess both mast cell stabilizing and gastrointestinal cytoprotective activity. These include zinc compounds, sodium cromoglycate, FPL 52694, ketotifen, aloe vera, certain flavonoids such as quercetin, some sulfated proteoglycans such as chondroitin sulfate and dehydroleucodine. Dehydroleucodine, a sesquiterpene lactone isolated from Artemisia douglasiana Besser, exhibits anti-inflammatory and gastrointestinal cytoprotective action. The lactone stimulates mucus production, and inhibits histamine and serotonin release from intestinal mast cells. The lactone could act as a selective mast cell stabilizer by releasing cytoprotective factors and inhibiting pro-inflammatory mast cell mediators.
Key words: Mast Cells. Mucosal Defense. Gastrointestinal. Dehydroleucodine.
I. Gastrointestinal mucosal defense
The term "gastrointestinal mucosal defense" refers to the combination of factors that allow the gastrointestinal mucosa to withstand exposure to substances with a wide range of pH, osmolarity, and temperature, solutions with detergent properties (e.g., bile), and bacterial products capable of eliciting local and systemic inflammatory reactions (Wallace and Granger, 1996; Wallace and Ma, 2001).
The mucosa is not impervious to damage by the various substances we eat and the endogenous secretions. Indeed, it is likely that mucosa injury occurs regularly. However, the mucosa can repair such injury quickly, thereby limiting it to the most superficial layer of cells and preventing entry into the systemic circulation of substances detrimental to the organism (Wallace and Granger, 1996; Wallace and Ma, 2001). The resistance of the mucosa can also be enhanced when irritants are present in the gastrointestinal tract. Thus, the ability of the mucosa to resist significant injury is attributable to a dynamic process rather than a static barrier (Wallace and Granger, 1996; Wallace and Ma, 2001).
The various components of mucosal defense can be viewed as being organized as a hierarchy, corresponding to the anatomical organization of the mucosa (Wallace and Granger, 1996; Wallace and Ma, 2001). The first level of defense consists of the factors secreted into the lumen including acid, bicarbonate, mucus, immunoglobulins and other antibacterial substances (e.g., lactoferrin), and surface-active phospholipids (Wallace and Granger, 1996; Wallace and Ma, 2001).
The second level of defense is the epithelium, which is remarkably resistant to acid-induced injury and forms a relatively tight barrier to passive diffusion (Wallace and Granger, 1996; Wallace and Ma, 2001). The epithelium is capable of undergoing extremely rapid repair if its continuity is disrupted.
The third level of defense is the mucosal microcirculation, in concert with sensory afferent nerves within the mucosa and submucosa (Wallace and Granger, 1996; Wallace and Ma, 2001). Back-difussion of acid or toxins into the mucosa results in a neurally mediated elevation of mucosal blood flow that is critical for limiting damage and facilitating repair.
The fourth level of defense is the mucosal immune system, consisting of various "alarm cells", such as the mast cell and macrophage, which sense entry of foreign material into the mucosa and orchestrate an appropriate inflammatory response (Wallace and Granger, 1996; Wallace and Ma, 2001). These cells sense the entry of foreign matter or antigen into the lamina propria, and respond by releasing soluble mediators and cytokines that initiate a defensive inflammatory response to prevent the foreign matter from gaining access to the systemic circulation (Wallace, 1996). Many of the inflammatory mediators and cytokines that are released exert chemotactic effects on leukocytes, resulting in their recruitment into the region where the immunocytes have been activated (Wallace, 1996).
The fifth level of mucosal defense is called into play when an ulcer has formed -an ulcer being defined as a break in mucosa that extends through the muscularis mucosae. In these circumstances, the ulcer is repaired through growth and re-development of gastric glands, tissue remodeling, growth of new blood vessels (angiogenesis), and re-innervation of the mucosa by the extrinsic and intrinsic nerves (Wallace, 2001). Enhanced numbers of inflammatory cells, mainly mast cells and eosinophils, are well known to occur during angiogenesis and tissue remodeling (Armetti et al., 1999).
The purpose of this review, based on studies from our laboratory as well as from others, is to summarize salient features of mast cell immunobiology and to describe their associations with gastrointestinal mucosal defense.
II. Mast cells
II.1. Morphology
Mast cells are round or elongated, mononuclear cells filled with secretory granules of variable size and shape (Figs. 1, 2 and 3); their diameter is approximately 10_20 mm (Mc Neil and Austen, 1995; Galli, 2000). Histochemically, mast cell granules exhibit metachromasia when stained with cationic dyes, such as toluidine blue. When the blue dye is taken up by the granules, there is a colour change from blue to pinkish-purple (Mc Neil and Austen, 1995; Galli, 2000). Ultrastructurally, mature mast cells have single-lobed nuclei, narrow regular surface folds, inconspicuous synthetic organelles, and numerous cytoplasmic granules (Figs. 2 and 3). The granules show multiple ultrastructural patterns: scroll granules, crystal granules, particle granules and mixed granules (Mc Neil and Austen, 1995; Galli, 2000). Certain populations of mast cells also contain large lipid bodies without the surrounding membrane (Massey et al., 1991).

Figure 1. Light micrographs of 1 µm sections of mouse duodenum stained with toluidine blue. Scale bar = 10 µm. A and C: control group. Typical mast cells (arrows) can be observed in proximity to blood (BV) and lymphatic (LV) vessels of the mucosal (A) and submucosal (C) layers. B and D: DhL group. Abundant mast cells (arrows) with closely packed cytoplasm granules can be observed in the mucosa (B) and submucosa (D). E: epithelium; G: Brunner´s gland.

Figure 2. Transmission electron micrographs showing mast cells in the duodenum of mice. A: Control submucosal mast cell. Numerous homogenously dense secretory granules distributed throughout the cell cytoplasm (arrow), and a regular and elongated nucleus can be observed (N). A small number of granules present an inhomogeneous matrix (arrowhead). Scale bar = 0.5 µm. B: DhL mucosal mast cell. Similar characteristics to A. Scale bar = 0.35 µm. C: DhL submucosal mast cell. Abundant swollen granules with a reduction in their homogeneity and varying degrees of electron density can be clearly observed (arrowhead). A small number of granules are homogenously dense (arrow). N: irregular nucleus with anfractuous surface. CF: collagen fibers. Scale bar = 0.5 µm. Inset: secretory granules with different degrees of electron density. Scale bar = 0.35 µm.
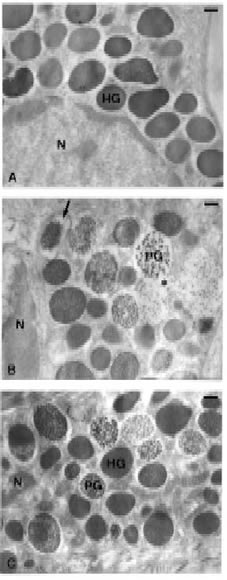
Figure 3. Transmission electron micrographs showing mast cells in the jejunal submucosa of mice. (A) Basal. Numerous homogenously dense secretory granules (HG) distributed throughout the cell cytoplasm can be clearly observed. (B) 48/80. Mast cells show obvious morphological changes and evidence of enhanced granule release compared with control cells. Enlargement of the space between the granule and its membrane (arrow), granule swelling, and reduced electron density of the granule contents are shown. Granules present a particulated appearance (particle type granules, PG) showing -from left to right- increasing degrees of swelling and particulation. The formation of intracytoplasmic channels (asterisk) can be seen. (C) DhL+48/80. Homogenously dense (HG) with no detectable space between the granule and its membrane, and particle type granules (PG) showing a space between the granule content and the perigranular membrane can be observed. Neither intracytoplasmic channels nor swelling can be seen. Scale bar = 0.3 µm. Figure from Penissi et al., 2003b.
II.2. Origin
Mast cells are derived from hematopoietic progenitor cells (Valent et al., 1991; Galli et al., 1995; Vliagoftis et al., 1997). With rare exceptions, mature mast cells are not identifiable in the blood; instead, the blood contains less differentiated mast cell progenitors. They continue their differentiation and maturation in the periphery, like the connective tissue or the serosa-lined cavities such as the peritoneal cavity, as has been described in mice and rats. It is now becoming clear that mast cells express the receptor for stem cell factor (SCF receptor or c-kit) that binds to SCF, a specific growth factor for mast cells. The interaction between SCF and c-kit is crucial for the growth and development of mast cells (Mc Neil and Austen, 1995; Galli, 2000). Morover, apparently "mature" mast cells in peripheral tissues can also express proliferative ability (Mc Neil and Austen, 1995; Galli, 2000).
II.3. Subtypes and heterogeneity
In humans, two types of mast cells have been described, based on structural, biochemical and functional data: 1) Those staining positive with anti-tryptase antibodies alone (designated MCT), and 2) Those staining with antibodies to tryptase and chymase (designated MCTC) (Schwartz et al., 1987; Church and Levi-Schaffer, 1997; Schwartz, 1998). The MCT mast cell expresses tryptase predominantly and is usually localized to mucosal surfaces in close relationship to T cells, especially of the Th2-type. The MCT is increased in allergic and parasitic diseases and diminished numbers are seen in HIV-infected patients (Church and Levi-Schaffer, 1997). Structurally, granules from MCT are scroll-rich. The MCTC mast cell, on the other hand, expresses tryptase, chymase, carboxypeptidase and cathepsin G. It predominates in the gastrointestinal tract as well as in skin, synovium and subcutaneous tissues. Increased numbers of MCTC mast cells are seen in fibrotic diseases while numbers are relatively unchanged in allergic or parasitic diseases and in HIV infection. MCTC mast cells have lattice and grating structures and are scroll-poor. Thus, while MCT mast cells are central to inflammation and immune-system related, with a primary role in host defense, MCTC mast cells may be more important to tissue remodeling and angiogenesis, rather than immunologic protection. Both types of mast cells express FceRI (the high affinity IgE receptor) and are involved in allergic type responses. The murine counterparts of these subtypes have been referred to as mucosal mast cells (MMC), resident at the epithelium and in the subepithelial lamina propria, and connective tissue mast cells (CTMC), found predominantly in the submucosa (Wershil and Galli, 1991; Mc Neil and Austen, 1995; Stenton et al., 1998). MMC and CTMC are histochemically, ultrastructurally, and functionally different. They also vary in their pharmacological properties, for example in their sensitivity to various secretagogues such as compound 48/80, and anti-allergic compounds such as sodium cromoglycate (Wershil and Galli, 1991; Mc Neil and Austen, 1995; Stenton et al., 1998).
II.4. Mediators
Mast cells represent a rich source of a variety of potent biologically active mediators. The mediators derived from mast cells are divided into two groups (Mc Neil and Austen, 1995; Galli, 2000; Mekori and Metcalfe, 2000). The first group includes those mediators that are preformed and stored in secretory granules, while the second group includes those mediators that are newly generated when mast cells become activated. Among the preformed mediators are histamine, serotonin, proteases and proteoglycans, and some cytokines like TNF-a (Gordon and Galli, 1990); and among the newly-generated mediators are the arachidonic acid metabolites leucotriene C4 and prostaglandin D2, the phospholipid derivative platelet-activating factor (PAF) and a variety of cytokines (Mc Neil and Austen, 1995).
III. Mast cells and gastrointestinal mucosal defense
Mast cells are important components of the normal architecture of the gastrointestinal tract. Changes in the number of mast cells at various anatomic sites, or evidence of activation of the cells for mediator release, have been observed in a wide spectrum of adaptive or pathologic immune responses, and in a large number of disease processes, many involving the gastrointestinal tract. Evidence such as this supports the notion that mast cells can substantially influence immunologic and pathologic processes in the gastrointestinal tract and may even affect certain normal functions of the stomach or intestine (Bischoff et al., 1996; Echtenacher et al., 1996; Wallace and Granger, 1996; Furuta et al., 1997; Bischoff et al., 2000; Wershil, 2000; Andoh et al., 2001; Kolaczkowska et al., 2001).
Many substances released from gastrointestinal mast cells are biologically active and mediate numerous processes: blood flow regulation, epithelial and endothelial permeability, mucosal secretion, gastrointestinal tract motility, immunological events related to the antigens of various origins, angiogenesis, and cancer development (Barczyk et al., 1995; Stenton et al., 1998). Growing evidence suggests physiological roles for intestinal mast cells in the protection of tissues from inflammatory damage, and in intestinal maturation (Barczyk et al., 1995; Stenton et al., 1998).
Gastrointestinal mast cells clearly play a role in many pathologic effects associated with food hypersensitivity (Stenton et al., 1998). Histamine and serotonin are valuable markers of mast cell activation (Theoharides et al., 1985; Glavin and Hall, 1991; Buckley and Coleman, 1992; Coelho et al., 1998; Bueno and Fioramonti, 1999; Jiang et al., 2000), and have been regarded as critical pathogenetic factors in the development of peptic ulcers (Cho, 1994; Myers et al., 1998). On the other hand, gastrointestinal mast cells appear to play important physiologic roles, such as in weaning (Stenton et al., 1998). Mast cells also play a protective role in defense against parasitic and microbial infections; thus, they have both positive and negative effects, but presently the mechanisms that control the balance of these various effects are poorly known (Galli and Wershil, 1996; Stenton et al., 1998). Various cytokines are involved in the regulation of mast cell function (Stenton et al., 1998). Nitric oxide and prostaglandins have been also identified as important mast cell mediators related to gastrointestinal mucosal protection (Stenton et al., 1998). Therefore, the activation of mast cells may produce either positive or negative effects in tissues depending on the mediators that are released. Morover, mast cell mediators can be differentially released according to the stimulus applied (Kops et al., 1990).
For example, nitric oxide (NO) released from gastrointestinal mast cells during degranulation can be either protective or deleterious in different disorders (Stenton et al., 1998). This depends on what type of nitric oxide synthase (NOS) is involved in these pathological conditions. Constitutive NOS (cNOS) is responsible for production of NO in physiological context. In contrast, inducible NOS (iNOS) produces NO in pathophysiological circumstances. NO is implicated in processes that maintain the integrity of the gastric epithelium. In this connection, it regulates gastric blood flow and directly stimulates gastric mucus secretion by activating soluble guanylate cyclase. A blockade of NO production resulted in an impairment of the vascular response and the subsequent alkaline flux in the lumen (Stenton et al., 1998; Cho, 2001; Tanaka et al., 2001). In addition, another protective role of NO is to depress the secretion of histamine, and possibly other mediators such as platelet activating factor (PAF), from mast cells (Barczyk et al., 1995).
Raud (1990) demonstrated that prostaglandins could partially suppress acute mast cell-dependent inflammation. With the use of isolated mast cells from the peritoneum and the intestinal mucosa, Hogaboam and associates (1993) demonstrated that several prostaglandins dose-dependently inhibited the release of mediators such as histamine, platelet-activating factor and TNF-a. The prostaglandins were found to be extremely potent modulators of mast cell reactivity. Inhibitory effects were observed at concentrations as low as 10-11 mol/L. The suppression of mast cell reactivity by prostaglandins may contribute to the well-documented cytoprotective effects of these agents (Robert, 1976).
Mast cells can also release cytokines, such as tumour necrosis factor-a (TNF-a), interleukin-4 (IL-4), interleukin-10 (IL-10) and interleukin-13 (IL-13), which were originally thought to contribute to inflammatory damage, but which may also have anti-inflammatory properties (Stenton et al., 1998). It has been reported that; in the gastrointestinal tract mast cell cytokines play roles in tissue protection (Stenton et al., 1998). For example, IL-4, IL-10 and IL-13 have anti-inflammatory properties such as the enhancement of ileal sodium and chloride absorption, inhibition of secretagogue-induced chloride secretion, and the ability to reduce the release of TNF-a by monocytes and macrophages (Stenton et al., 1998). TNF-a can be both inflammatory and anti-inflammatory under different conditions. It has been shown that one of the cytoprotective actions of TNF-a, which is released in few seconds after different stimuli, is the production of intestinal mucus secretion (Arnold et al., 1993).
IV. Mast cell stabilizers and gastrointestinal mucosal defense
Considerable effort throughout the 1990's has focused on the identification of new gastroprotective molecules. Some synthetic studies have been aimed at the preparation of new prostaglandins, prostacyclin mimetics, and thromboxane antagonists (Ares and Outt, 1998). New histamine H2 receptor antagonists have also been developed which, unlike cimetidine or ranitidine, now appear to couple true gastroprotective activity with antisecretory properties (Ares and Outt, 1998). One new H2 antagonist, ebrotidine, has shown clinical utility in preventing NSAID gastropathy (Ares and Outt, 1998). Many other types of structures (flavonoids, peptides, terpenoids, xanthines, and others), as well as compounds displaying certain pharmacological actions (5-hydroxytryptamine receptor binding, adrenergic receptor binding, mast cell stabilization, and others) have been linked in some way to gastroprotection (Ares and Outt, 1998).
It has been suggested that stabilization of mast cells may be a key mechanism to protect the gastrointestinal tract from injury (Karmeli et al., 1991; Eliakim et al., 1992; Hogaboam et al., 1993; Whittle, 1993; Low et al., 1995; Kalia et al., 2000; Kiraly et al., 2000; Ruh et al., 2000). Few molecules are known to possess both mast cell stabilizing and gastrointestinal cytoprotective activity. These include zinc compounds (Cho and Ogle, 1992), sodium cromoglycate (Cho and Ogle, 1992), FPL 52694 (Cho and Ogle, 1992), ketotifen (Cho and Ogle, 1992), aloe vera (Ro et al., 2000), certain flavonoids such as quercetin (Middleton et al., 2000), some sulfated proteoglycans such as chondroitin sulfate (Theoharides et al., 1999; Hori et al., 2001) and dehydroleucodine, a sesquiterpene lactone (Giordano et al., 1990; Penissi et al., 2003a,b).
V. Intestinal mast cells in response to dehydroleucodine
One of the fairly well documented preparations in traditional medicine is an infusion of the leaves of Artemisia douglasiana Besser, popularly known as "matico". Dehydroleucodine (DhL), a sesquiterpene lactone isolated from Artemisia douglasiana Besser (Giordano et al., 1990), prevents dose-dependently gastrointestinal damage in response to necrosis-inducing agents such as absolute ethanol (Piezzi et al., 1995; Penissi et al., 1998; Penissi et al., 2000).
The effects of DhL on the intestinal mast cell population have been studied by Penissi et al. (2003a) with the goal of testing the hypothesis that DhL induces changes in these cells probably related to its mechanism of gastrointestinal cytoprotection. No changes in the number and cytological structure of mucosal mast cells are induced after DhL treatment in the small intestine. However, the lactone itself significantly increases the number of mast cells of the submucosal layer, as well as their morphology and the distribution of their secretory granules. Thus, the intestinal mast cell population (mucosal and submucosal mast cells) exhibit a different response to the cytoprotective agent (Figs. 1 and 2).
Taking into account that in the small intestine the sole source of histamine is the mast cell (Cho, 1994), the effect of DhL on endogenous histamine content was also analyzed. As expected, this study showed that DhL -administered orally- increases histamine levels in duodenum, suggesting that the tissue histamine increase is related to the higher mast cell population found in the submucosal layer (Penissi et al., 2003a).
Although interesting, the finding of increased submucosal mast cell number induced by DhL raised several obvious questions. Firstly, which is the probable mechanism that mediates such an increase? It seems surprising that there is a significant increase in submucosal mast cell numbers and histamine levels in the small intestine within 60 min of treatment with DhL. For this reason, it is difficult to ascribe these changes to mast cell differentiation from bone marrow mast cell precursors. It is believed that DhL could induce mast cell migration from other anatomic sites. Secondly, why does mast cell number increase? Intestinal mast cells clearly play a role in many pathologic effects associated with food hypersensitivity (Stenton et al., 1998). However, mast cells also play a protective role in mucosal defense; thus, they have both positive and negative effects but, at present, the mechanisms that control the balance of these various effects are poorly known (Stenton et al., 1998). The authors think that the increased mast cell population observed in this study serves to enhance the production of cytoprotective mediators, such as NO or TNF-a. There is strong evidence in support of this possibility. Interestingly, in previous work it has been demonstrated that DhL prevents gastrointestinal damage elicited by necrosis-inducing agents such as absolute ethanol (Giordano et al., 1990; Piezzi et al., 1995; Penissi et al., 1998). It has also reported that this protective effect is related to the ability of the drug to stimulate mucus production (Penissi et al., 1998; Penissi and Piezzi, 1999). It has also been demonstrated that DhL exhibits strong anti-inflammatory action in acute and chronic models of experimental inflammation (Guardia et al., 1995; Juárez et al., 1996). In addition, it has been demonstrated that the gastric cytoprotective effect of DhL is antagonized by NG-nitro-arginine, a NOS inhibitor, suggesting that NO is involved in the gastroprotection induced by DhL (Maria et al., 1998). Based on these previous reports, the cytoprotective action of DhL could probably be mediated by mast cell cytoprotective mediators, such as NO and/or cytokines. This possibility is also supported by another finding: DhL-treated submucosal mast cells exhibit secretory granules with reduced electron density and different degrees of particulation. Some authors (Lawson et al., 1977; Chock, 1988) have described that -at the transmission electron microscope level- mast cell mediator release is associated with readily detectable alterations in the ultrastructural appearance of the granule, including swelling and reduced electron density of the granule contents. The obvious mast cell ultrastructural changes induced by DhL suggest that the lactone is able to promote mediator release from intestinal mast cells. However, the authors also thought that the lactone probably inhibits the release of inflammatory mediators from enteric mast cells, acting thus as a mast cell stabilizer in response to injury. This reasoning was based on the cytoprotective and anti-inflammatory activity of DhL, which argued against the possibility that swollen and particulated granules detected in the preparations could have been due to release of pro-inflammatory mediators such as histamine.
The effect of DhL on compound 48/80-induced histamine and serotonin release in the isolated mouse jejunum was evaluated (Penissi et al., 2003b). It has been established that this mast cell secretagogue causes mucosal injury in the gastrointestinal tract and mast cell mediator release (such as histamine and serotonin), and that released histamine and serotonin play a role in mediating the mucosal injury (Ohta et al. , 1997; Boros et al., 1999). Interestingly, incubation of the jejuna with a 10 mg/ml compound 48/80 solution increased histamine and serotonin release, and that this effect was inhibited by DhL.
The effect of DhL on mast cell morphology by light and electron microscopy was also analyzed (Penissi et al., 2003b). In this study, the significantly increased histamine and serotonin release after compound 48/80 treatment was closely associated with a reduction in the number of granulated submucosal mast cells and with obvious mast cell ultraestructural changes (Fig. 3). The morphological findings also showed that DhL inhibited the reduction in the number of granulated metachromatically stained submucosal mast cells, suggesting an interaction of the lactone with mast cell population and an inhibition of the degranulation induced by compound 48/80. Furthermore, DhL induced some ultrastructural mast cell changes, but this last action was less dramatic than that elicited by compound 48/80 itself.
Morover, DhL inhibited compound 48/80-induced serotonin release from rat purified peritoneal mast cells (Penissi et al., 2002).
Some authors have described that a reduction in the number of granulated mast cells in the gastrointestinal tract after compound 48/80 treatment is due to increased degranulation (Saavedra-Delgado et al., 1984; Boros et al., 1999a,b). Other authors have shown that -at the transmission electron microscope level- granules from unreleased mast cells are present as homogeneous, dense-staining cytoplasmic inclusions surrounded by their granule membranes (Lawson et al., 1977). It has been also described that mast cell mediator release is associated with readily detectable alterations in the ultraestructural appearance of the granule, including the enlargement of the space between the granule and its membrane, granule swelling, reduced electron density of the granule contents, and the presence of a network of cavities (Lawson et al., 1977).
The fact that DhL inhibits compound 48/80-induced histamine and serotonin release from mast cells in the isolated mouse jejunum and in rat purified peritoneal mast cells, raises the possibility that the lactone acts as a mast cell stabilizer in the intact animal. Thus, DhL could prevent histamine and serotonin release and, consequently, intestinal damage elicited by necrosis-inducing agents such as compound 48/80. This mechanism is quite analogous to the action of ketotifen, a mast cell stabilizer that significantly protects the gastrointestinal mucosa against lesions induced by necrotizing agents, histamine or compound 48/80 (Karmeli et al., 1991; Eliakim et al., 1992; Kiraly et al., 2000; Ruh et al., 2000). The comparison between the effect of DhL and classically used mast cell stabilizer such as ketotifen is showed in Figure 4.

Figure 4. Effect of DhL and ketotifen (0.8, 1.6 and 3.2 mmol/l) on compound 48/80-histamine release. Results were expressed as pg histamine released over 90 min/mg wet tissue weight. All values are presented as means ± SEM. Figure from Penissi et al., 2003b.
Summarizing, on the one hand, it has shown that DhL inhibited the basal and compound 48/80-induced histamine release from intestinal and peritoneal mast cells (Penissi et al., 2003b). By the other hand, it has been previously demonstrated that the intestinal mucosa treated only with DhL showed normal characteristics, no morphological damage to the villi, and an enhancement of defensive intestinal factors such as mucus secretion (Fig. 5). In conclusion, DhL, in addition to its specific action on the submucosal mast cell population (Penissi et al., 2003a), could act as a selective mast cell stabilizer by releasing cytoprotective factors and inhibiting pro-inflammatory mast cell mediators. These possible effects can be also explained on the basis of results by Kops et al. who reported that, depending on the applied stimuli, mediators can be released differentially from mast cells (Kops et al., 1990).

Figure 5. Mechanisms of the cytoprotective action of dehydroleucodine at the gastrointestinal level.
References
Andoh A, Fujiyama Y, Araki Y, Kimura T, Tsujikawa T, Bamba T (2001). Role of complement activation and mast cell degranulation in the pathogenesis of rapid intestinal ischemia/reperfusion injury in rats. Digestion. 63: 103-107. [ Links ]
Ares JJ, Outt PE (1998). Gastroprotective agents for the prevention of NSAID-induced gastropathy. Curr Pharm Des. 4: 17-36. [ Links ]
Armetti L, Admon D, Solomon A, Levi-Schaffer F (1999). Mast cells and eosinophils-Their role in tissue remodeling. ACI International. 11: 22-28. [ Links ]
Arnold JW, Klimpel GR, Niesel DW (1993). Tumour necrosis factor (TNF alpha) regulates intestinal mucus production during salmonellosis. Cell Immunol. 151: 336-344. [ Links ]
Barczyk M, Debek W, Chyczewski L (1995). Mast cells in the gastrointestinal tract. Rocz Akad Med Bialymst. 40: 26-57. [ Links ]
Bischoff SC, Mayer JH, Manns MP (2000). Allergy and the gut. Int Arch Allergy Immunol. 121: 270-283. [ Links ]
Bischoff SC, Wedemeyer J, Herrmann A, Meier PN, Trautwein C, Cetin Y, Maschek H, Stolte M, Gebel M, Manns MP (1996). Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 28: 1-13. [ Links ]
Boros M, Kaszaki J, Ordogh B, Nagy S (1999a). Mast cell degranulation prior to ischemia-reperfusion injury in the canine small intestine. Inflamm Res. 48: 193-198. [ Links ]
Boros M, Ordogh B, Kaszaki J, Nagy S (1999b). The role of mast cell degranulation in ischaemia-reperfusion-induced mucosal injury in the small intestine. Ann Acad Med Singapore. 28: 79-84. [ Links ]
Buckley MG, Coleman JW (1992). Cycloheximide treatment of mouse mast cells inhibits serotonin release. Biochem Pharmacol. 44: 659-664. [ Links ]
Bueno L, Fioramonti J (1999). Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol. 13: 42A-46A. [ Links ]
Cho CH (1994). The role of endogenous ulcerogenic mediators in the pathogenesis of peptic ulcer. Life Sci. 55: 1521-1535. [ Links ]
Cho CH (2001). Current roles of nitric oxide in gastrointestinal disorders. J Phisiol Paris. 95: 253-256. [ Links ]
Cho CH, Ogle CW (1992). The pharmacological differences and similarities between stress- and ethanol-induced gastric mucosal damage. Life Sci. 51: 1833-1842. [ Links ]
Chock SP, Schmauder-Chock EA (1988). Synthesis of prostaglandins and eicosanoids by the mast cell secretory granule. Biochem Biophys Res Commun. 156: 1308-1315. [ Links ]
Church MK, Levi-Schaffer F (1997). The human mast cell. J Allergy Clin Immunol. 99: 155-160. [ Links ]
Coelho AM, Fioramonti J, Bueno L (1998). Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci. 43: 727-737. [ Links ]
Echtenacher B, Mannel DN, Hultner L (1996). Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 381: 75-77. [ Links ]
Eliakim R, Karmeli F, Okon E, Rachmilewitz D (1992). Ketotifen effectively prevents mucosal damage in experimental colitis. Gut. 33: 1498-1503. [ Links ]
Furuta GT, Schmidt-Choudhury A, Wang MY, Wang ZS, Lu L, Furlano RI, Wershil BK (1997). Mast cell-dependent tumor necrosis factor alpha production participates in allergic gastric inflammation in mice. Gastroenterology. 113: 1560-1569. [ Links ]
Galli SJ (2000). Mast cells and basophils. Curr Opin Hematol. 7: 32-39. [ Links ]
Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ (1995). Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 107: 51-53. [ Links ]
Galli SJ, Wershil BK (1996). The two faces of the mast cell. Nature. 381: 21-22. [ Links ]
Giordano OS, Guerreiro E, Pestchanker MJ (1990). The gastric cytoprotective effect of several sesquiterpene lactones. J Nat Prod. 53: 803-809. [ Links ]
Glavin GB, Hall AM (1991). Experimental gastric ulcer: Recent data suggest potential new therapeutic strategies. Asia Pacific J Pharmacol. 6: 331-339. [ Links ]
Gordon JR, Galli SJ (1990). Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 346: 274-276. [ Links ]
Guardia T, Guzmán JA, Juárez A, Guerreiro E, Giordano OS (1995). Acción antiinflamatoria de dehidroleucodina (DhL). Acta Physiol Pharmacol Ther Latinoam. 45: 226. [ Links ]
Hogaboam CM, Bissonnette EY, Chin BC, Befus D, Wallace JL (1993). Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterology. 104: 122-129. [ Links ]
Hori Y, Hoshino J, Yamazaki C, Sekiguchi T, Miyauchi S, Horie K (2001). Effects of chondroitin sulfate on colitis induced by dextran sulfate sodium in rats. Jpn J Pharmacol. 85: 155-160. [ Links ]
Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D (2000). 5-HT (3) and histamine H (1) receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 119: 1267-1275. [ Links ]
Juárez AD, Guardia T, Guzmán JA, Giordano OS, Guerreiro E (1996). Antiinflammatory activity of dehydroleucodine (DhL) in rats. Acta Physiol Pharmacol Ther Latinoam. 46: 194. [ Links ]
Kalia N, Bardhan KD, Reed MW, Jacob S, Brown NJ (2000). Mast cell stabilization prevents ethanol-induced rat gastric mucosal injury: mechanisms of protection. J Gastroenterol Hepatol. 15: 133-141. [ Links ]
Karmeli F, Eliakim R, Okon E, Rachmilewitz D (1991). Gastric mucosal damage by ethanol is mediated by substance P and prevented by ketotifen, a mast cell stabilizer. Gastroenterology. 100: 1206-1216. [ Links ]
Kiraly A, Suto G, Tam B, Hermann V, Mozsik G (2000). Vagus-mediated activation of mucosal mast cells in the stomach: effect of ketotifen on gastric mucosal lesion formation and acid secretion induced by a high dose of intracisternal TRH analogue. J Physiol Paris. 94: 131-134. [ Links ]
Kolaczkowska E, Seljelid R, Plytycz B (2001). Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1mice. J Leukoc Biol. 69: 33-42. [ Links ]
Kops SK, Theoharides TC, Cronin CT, Kashagian MG, Askenase PW (1990). Ultraestructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tissue Res 262: 415-420. [ Links ]
Lawson D, Raff MC, Gomperts B, Fewtrell C, Gilula NB (1977). Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 72: 242-259. [ Links ]
Low J, Grabow D, Sommers C, Wallace J, Lesch M, Finkel M, Schrier D, Metz A, Conroy MC (1995). Cytoprotective effects of CI-959 in the rat gastric mucosa: Modulation of leukocyte adhesion. Gastroenterology. 109: 1224-1233. [ Links ]
Maria AO, Wendel GW, Guzmán JA, Giordano OS, Guerreiro E (1998). Gastric cytoprotective activity of dehydroleucodine in rats. Role of nitric oxide. Pharmacol Res. 37: 281-284. [ Links ]
Massey WA, Guo CB, Dvorak AM, Hubbard WC, Bhagavan BS, Cohan VL, Warner JA, Kagey-Sobotka A, Lichtenstein LM (1991). Human uterine mast cells. Isolation, purification, characterization, ultrastructure, and pharmacology. J Immunol. 147: 1621-1627. [ Links ]
Mc Neil HP, Austen KF (1995). Biology of the mast cell. In: Samter´s Immunologic Diseases. MM Frank, KF Austen, HN Claman, ER Unanue ER, Eds. Little, Brown and Company, New York, pp. 185-204. [ Links ]
Mekori YA, Metcalfe DD (2000). Mast cells in innate immunity. Immunol Rev. 173: 131-140. [ Links ]
Middleton E, Kandaswami C, Theoharides TC (2000). The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol Rev. 52: 673_751. [ Links ]
Myers CP, Hogan D, Yao B, Koss M, Isenberg JI, Barrett KE (1998). Inhibition of rabbit duodenal bicarbonate secretion by ulcerogenic agents: histamine-dependent and _independent effects. Gastroenterology. 114: 527-535. [ Links ]
Ohta Y, Kobayashi T, Nishida K, Ishiguro I (1997). Relationship between changes of active oxygen metabolism and blood flow and formation, progression, and recovery of lesions in gastric mucosa of rats with a single treatment of compound 48/80, a mast cell degranulator. Dig Dis Sci. 42: 1221-1232. [ Links ]
Penissi AB, de Rosas JC, Fogal T, Mariani ML, Nicovani S, Rudolph MI, Piezzi RS (2002). Dehidroleucodina inhibe la degranulación de mastocitos peritoneales inducida por el compuesto 48/80. Medicina. 62: 513. [ Links ]
Penissi AB, Fogal T, Guzmán JA, Piezzi RS (1998). Gastroduodenal mucosal protection induced by dehydroleucodine. Mucus secretion and role of monoamines. Dig Dis Sci. 43: 791-798. [ Links ]
Penissi AB, Mariani ML, Souto M, Guzmán JA, Piezzi RS (2000). Changes in gastroduodenal 5-hydroxytryptamine-containing cells induced by dehydroleucodine. Cells Tissues Organs. 166: 259-266. [ Links ]
Penissi AB, Piezzi RS (1999). Effect of dehydroleucodine on mucus production. A quantitative study. Dig Dis Sci. 44: 708-712. [ Links ]
Penissi AB, Rudolph MI, Fogal T, Piezzi RS (2003a). Changes in duodenal mast cells in response to dehydroleucodine. Cells Tissues Organs. 173: 234-241. [ Links ]
Penissi AB, Rudolph MI, Villar M, Coll RC, Fogal TH, Piezzi RS (2003b). Effect of dehydroleucodine on histamine and serotonin release from mast cells in the isolated mouse jejunum. Inflamm Res. 52: 199-205. [ Links ]
Piezzi RS, Guzmán JA, Guardia T, Pestchanker MJ, Guerreiro E, Giordano OS (1995). Dehydroleucodine prevents ethanol-induced necrosis in the mice duodenal mucosa. A histological study. Biocell 19: 27-33. [ Links ]
Raud J (1990).Vasodilatation and inhibition of mediator release represent two distinct mechanisms for prostaglandin modulation of acute mast cell-dependent inflammation. Br J Pharmacol. 99: 449-54. [ Links ]
Ro JY, Lee BC, Kim JY, Chung JY, Chung MH, Lee SK, Jo TH, Kim KH, Park YI (2000). Inhibitory mechanism of Aloe single component (alprogen) on mediator release in guinea pig lung mast cells activated with specific antigen-antibody reactions. JPET. 292: 114_121. [ Links ]
Robert A (1976). Antisecretory, antiulcer, cytoprotective and diarrheogenic properties of prostaglandins. Adv Prost Thrombox Res. 2: 507-20. [ Links ]
Ruh J, Vogel F, Schmidt E (2000). In vivo assessment of villous microcirculation in the rat small intestine in indomethacin-induced inflammation: role of mast-cell stabilizer ketotifen. Acta Physiol Scand. 170: 137-143. [ Links ]
Saavedra-Delgado AM, Turpin S, Metcalfe DD (1984). Typical and atypical mast cells of the rat gastrointestinal system: distribution and correlation with tissue histamine. Agents Actions. 14: 1-7. [ Links ]
Schwartz LB (1998). The Mast Cell. In: Textbook of Rheumatology. W.N. Kelley, E.D. Harris, S. Ruddy and C.B. Sledge, Eds. W.B. Saunders Company, Philadelphia, pp. 161-175. [ Links ]
Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM (1987). Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 138: 2611-2615. [ Links ]
Stenton GR, Vliagoftis H, Befus D (1998). Role of intestinal mast cells in modulating gastrointestinal pathophysiology. Ann Allergy Asthma Immunol. 81: 1-15. [ Links ]
Tanaka A, Mizoguchi H, Kunikata T, Miyazawa T, Takeuchi K (2001). Protection by constitutively formed nitric oxide of intestinal damage induced by indomethacin in rats. J Physiol Paris. 95: 35-41. [ Links ]
Theoharides TC, Kops SK, Bondy PK, Askenase PW (1985). Differential release of serotonin without comparable histamine under diverse conditions in the rat mast cell. Biochem Pharmacol. 34: 1389-1398. [ Links ]
Theoharides TC, Letourneau R, Patra P, Hesse L, Pang X, Boucher W, Mompoint C, Harrington B (1999). Stress-induced rat intestinal mast cell intragranular activation and inhibitory effect of sulfated proteoglycans. Dig Dis Sci. 44: 87S-93S. [ Links ]
Valent P, Sillaber C, Bettelheim P (1991). The growth and differentiation of mast cells. Prog Growth Factor Res. 3: 27-41. [ Links ]
Vliagoftis H, Worobec AS, Metcalfe DD (1997). The protooncogene c-kit and c-kit ligand in human disease. J Allergy Clin Immunol. 100: 435-440. [ Links ]
Wallace JL (1996). Cooperative modulation of gastrointestinal mucosal defence by prostaglandins and nitric oxide. Clin Invest Med. 19: 346-51. [ Links ]
Wallace JL (2001). Mechanisms of protection and healing: current knowledge and future research. Am J Med. 110: S19_S23. [ Links ]
Wallace JL, Granger DN (1996). The cellular and molecular basis of gastric mucosal defense. FASEB J. 10: 731-740. [ Links ]
Wallace JL, Ma L (2001). Inflammatory Mediators in Gastrointestinal Defense and Injury. Exp Biol Med. 226: 1003-1015. [ Links ]
Wershil BK (2000). Mast cell-deficient mice and intestinal biology. Am J Physiol Gastrointest Liver Physiol. 278: G343-G348. [ Links ]
Wershil BK, Galli SJ (1991). Gastrointestinal mast cells. New approaches for analyzing their function in vivo. Gastroenterology Clinics of North America. 20: 613-627. [ Links ]
Whittle B (1993). Modulation by prostanoids of the release of inflammatory mediators from mast cells: involvement in mucosal protection?Gastroenterology. 104: 314-317. [ Links ]
Received on June 20, 2003.
Accepted on July 18, 2003.














