Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.27 n.3 Mendoza ago./dic. 2003
Sperm ultrastructure and spermatogenesis in the lizard, Tropidurus itambere
Adelina Ferreira and Heidi Dolder
Departamento de Biologia Celular, Universidade Estadual de Campinas (Unicamp), Brazil
Address correspondence to: Dr. Heidi Dolder. Departamento de Biologia Celular, Universidade Estadual de Campinas. CEP 13084971 Campinas, SP, BRAZIL. Fax: (55-19) 3788 6111; E-mail: heidi@unicamp.br
Abstract: Spermatogenesis, with emphasis on spermiogenesis, is described for the lizard, Tropidurus itambere, using light microscopy, phase contrast and epifluorescence, as well as scanning and transmission electron microscopy. Cellular differentiation involves events of chromatin condensation, nuclear elongation and the formation of structural complexes, such as the acrosomal and axonemal ones. Other new characteristics, exclusive for this species, include various aspects of the subacrosomal granule, the insertion of the pro-acrosomal vesicle and the development of these structures to participate in the acrosomal complex. Radial projections occur just above the nuclear shoulders, which have been recognized already from the beginning of cellular elongation. The development of the midpiece, the dense bodies, formation of the flagellum and elimination of residual cytoplasm result in the final characterization of the mature spermatozoon. Comparisons between Tropiduridae and other lizard families are made.
Keywords: Ultrastructure. Sperm. Spermatogenesis. Lizard. Tropiduridae.
Introduction
The Tropidurus itambere (Squamata, Reptilia) is a species that occurs in the dense Atlantic forest domains, as well as open formations of the states of São Paulo and Minas Gerais, Brazil (Rodrigues, 1987). The adult male individuals present an annual reproductive cycle of the continuous type with variations in their reproductive activity. These animals are potentially fertile during every month of the year, except March, the month where a considerable reduction occurs in the testicular volume and consequently in the production of spermatozoa (Van Sluys, 1993). Ultrastructural studies of the mature spermatozoon are known for other lizards, as in Chamaleonidae (Jamieson, 1995; Oliver et al., 1996); Polychrotidae (Furieri, 1974; Teixeira et al., 1999; Scheltinga et al., 2001); Phrynosomatidae (Scheltinga et al., 2000) and also in Tropiduridae (Furieri, 1974; Teixeira et al., 1999). Some descriptions focus on phylogenetic relationships. Spermiogenesis is described for some Chamaleonidae lizards (Al-Hajj et al., 1987; Dehlawi and Ismail, 1990; Dehlawi et al., 1992), a few representatives of Iguanidae (Saita et al., 1988; Ferreira and Dolder, 2002), in Phrynosomatidae and Polychrotidae (Clark, 1967). Despite descriptions of spermiogenesis for the Iguania group, only Tropidurus torquatus, among the lizards of the Tropiduridae family has been detailed in the literature (Furieri, 1974; Cruz-Landim and Cruz-Höfling, 1977; Cruz-Höfling and Cruz-Landim, 1978; Vieira et al., 2001). The Tropidurus lizards were considered as pertaining to the Iguanidae family up to a recent systematic revision, which constituted the Tropiduridae family (Rodrigues, 1987). The possibility of contributing to the studies of phylogenetic relationships justifies detailed comparisons between these families. Data on spermatogenesis is uncommon for the majority of vertebrates, and almost absent for lizards. Some articles entitled spermatogenesis in lizards (Cruz et al., 1994; Martinage et al., 1996; Ibarguengoytia and Cussac, 1999; Amat et al., 2000) bring information only on a given structure during a particular phase of the reproductive cycle. Our objective is to confirm this data and to evaluate the ultrastructural particularities of spermatogenesis in Tropidurus itambere, as well as of the characteristics of its testicular spermatozoa.
Material and methods
The adult T. itambere (N=16) used in this study was collected from the Valinhos region (23o00' S, 47o00' W), São Paulo state, Brazil.
Light microscopy
Tissues were fixed overnight at 4oC in a solution containing 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer, pH 7.2, dehydrated in acetone and embedded in LRWhite resin. The sections stained with hematoxylin and eosin, were observed with a photomicroscope. A sperm suspension was spread and fixed as above. After drying at room temperature, it was observed with phase contrast. Some of these preparations were then stained for 15 min with 0.2 mg/ml 4,6-diamino-2-phenylindole (DAPI) in PBS buffer, washed, and mounted with Vectashield. They were examined with epifluorescence microscopy (Olympus, BX60), equipped with a BP 360-370nm excitation filter.
Scanning electron microscopy
Testis was fixed as above; subsequently, they were washed in buffer, imbibed in sucrose solution (0.5M-3M) and cryofractured in liquid nitrogen. They were washed in buffer and post-fixed for 1 h in 1% osmium tetroxide in 0.1M sodium caccodylate buffer, pH 7.2. The testes were dehydrated in acetone, critical point dried and sputter-coated with gold. They were observed with a scanning electron microscope (Jeol JSM 5800LV).
Transmission electron microscopy
Testis were fixed and embedded in LRWhite resin, as above. The ultrathin sections were stained with uranyl acetate and lead citrate and observed with the transmission electron microscope (Zeiss, Leo 906).
Results
During the peak reproductive period, all phases of spermatogenesis are observed (Fig. 1A). The germ cells present a regular organization, with the basally located spermatogonia, together with spermatocytes, occupying half of the layer of the spermatogenic epithelium (Figs. 1A, 1B). Next to the lumen a great amount of spermatids and spermatozoa are embedded in the cytoplasmic prolongations of the Sertoli cells (Fig. 1C). Spermatogonia present a large, central nucleus, with dense clumps of chromatin (Fig. 1D). Some mitotic figures can be found. However, the most commonly observed phases are anaphase and telophase, where the nuclear material is still condensed in chromosomes and the spindle microtubules can be found (Fig. 1D). Spermatocytes are rich in organelles, especially the Golgi complex. The nucleus is central, with loose chromatin (Fig. 1E).
The differentiation of spermatids into spermatozoa involves the events of nuclear elongation, formation of the acrosomal and axonemal complexes, and elimination of residual cytoplasm. Early spermatids possess a central, rounded nucleus, with granular chromatin and numerous mitochondria (Fig. 1F). The Golgi complex and the endoplasmic reticulum are well developed; the endoplasmic reticulum aggregates in patches (Fig. 2B).

Figure 1. A. Light microscopy. Seminiferous tubules showing the interstitial tissue (it), Sertoli cell (s), spermatogonia (g), spermatocytes (c), spermatids (t) and spermatozoa (z). Bar: 20 µm. B. and C. Scanning electron microscopy. B. Seminiferous tubules where germ cells are organized in tufts (*).Bar: 20 µm. C. Higher magnification of a group of spermatozoa (z) embedded in a Sertoli cell (s). Bar: 1.85 µm. D. _ F. Transmission electron microscopy. D. Group of spermatogonia and spermatocytes in various division stages (arrow). Bar: 1.92 µm. E. Groups of spermatocytes, with a large nucleus and many organelles such as the Golgi complex (G) and mitochondria (m). Notice the presence of cytoplasmic bridges (arrow head). Bar: 1.78 µm. F. Early spermatids with more compact chromatin and cytoplasm rich in endoplasmic reticulum (er) and mitochondria (m). The pro-acrosomal vesicle is in an initial stage (av). Bar: 1.78 µm.

Figure 2. Transmission electron microscopy. A. Early spermatid, with a large nucleus and loose chromatin. The axonemic complex is in the first developmental steps, with the proximal centriole (pc), distal centriole (dc) and annulus (an) identified. Bar: 1 µm. B. Higher magnification of the organelles that contribute to the formation of the pro-acrosomal vesicle (av): Golgi complex (g) and endoplasmic reticulum (er). Bar: 1 µm. C. An early spermatid shows a large pro-acrosomal vesicle (av), with the acrosomal granule in its interior (ag). Notice a more evident chromatin condensation around the vesicle's depression into the nucleus (arrow heads). Bar: 1 µm. D. A more advanced early spermatid presents chromatin in initial condensation. Structures between nucleus (n) and pro acrosomal vesicle (av) will originate the acrosomal complex including an electron-dense region that will develop into the subacrosomal cone (sc), and a clear region, precursor of the epinuclear electron-lucent region (et). In the opposite cell pole, structures that will originate the axonemic complex, such as the proximal centriole (pc), the distal centriole (dc) and the annulus (an), can be found. Bar: 1 µm. E. - F. Stages in chromatin condensation. The curved arrows indicate the helical arrangement of the microtubules or the chromatin, respectively. Bar: 0.25 µm. G. Elongating spermatid, with compacting chromatin (curved arrow), surrounded by manchette microtubules (mt). The acrosomal complex now includes the subacrosomal cone (sc), a large subacrosomal clear zone (cz) and the external acrosome (a). Bar: 0.28 µm.
To constitute the acrosomal complex, innumerable vesicles dispersed in the cytoplasm, formed in the Golgi complex, accumulate beside the nucleus, and fuse to form the pro-acrosomal vesicle, which is lodged in a large nuclear depression (Figs. 1F, 2C, 2D). The vesicle begins to flatten, containing a loose clear material (Fig. 2D). As the pro-acrosomal vesicle develops, a dense granule appears in its interior. This granule is initially attached to the vesicle membrane, in contact with the nucleus (Fig. 2C). Between the nucleus and the vesicle, an electron dense layer is observed (Figs. 2D, 2G). Between these layers, two other clear layers can be found that will form the epinuclear electron-lucent region and the subacrosomal clear zone (Figs. 2D, 2G). Chromatin adjacent to the vesicle becomes more compact than the remaining nuclear content (Fig. 2C).
The acrosomal complex begins to take on its final structure covering the initial narrowed portion of the nucleus (Figs. 2G, 3A, 3B, 3C). Two main compartments constitute this complex: the acrosome and the perforatorium. In the acrosome, two compartments can be further identified: an external acrosomal vesicle and an internal subacrosomal cone (Figs. 2G, 3C, 3F, 3G, 3H). The acrosomal vesicle is made up of a clear cortex and an electron dense matrix (Figs. 3C, 3D). The subacrosomal cone is well developed, circular and homogenous in cross section (Figs. 2G, 3F, 3G, 3H). Just above the nuclear tip and beneath the subacrosomal cone, is the epinuclear electron-lucent region (Figs. 3C, 3F, 3H). The perforatorium is a short rod located be tween acrosome and nucleus (Figs. 3D, 3E) partly surrounded by the subacrosomal cone. Separating the perforatorium and the vesicular acrosome is a clear layer identified as the subacrosomal clear zone (Figs. 3C, 3D, 3E, 3F, 3G, 3H).
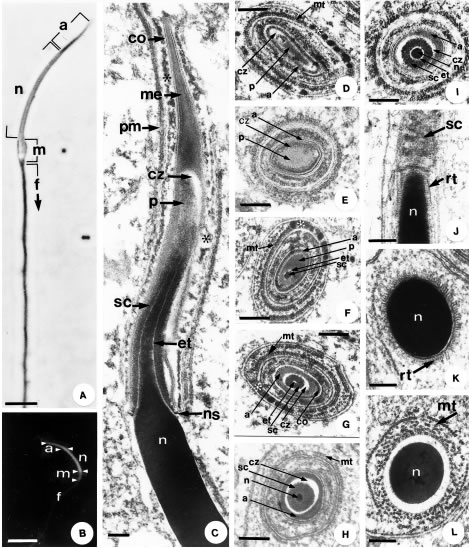
Figure 3. A. Phase contrast microscopy of the mature spermatozoon. The acrosome (a) corresponds to the darker, well-formed region at the nuclear apex (n). In the midpiece (m), it is possible to observe a density variation due to the dense bodies between the mitochondria. Flagellum, (f). Bar: 2.5 µm. B. Epifluorescence microscopy shows the acrosome (a) corresponding to a less evident tip, the nucleus (n) to the most brilliant portion, followed by the midpiece (m). Bar: 5 µm. Notice in A. and B. the curvature of the head. C _ L. Transmission electron microscopy. C. Longitudinal section of the spermatozoon head, with a compact, very electron-dense nucleus (n). Inserted over the apex of the nucleus, the acrosomal complex is made up of different regions. Bar: 1 µm. D. - I. Progressive transverse sections of the acrosomal complex. Bar: 0.30 µm. J. Longitudinal section of the transition region between the acrosomal complex and the nucleus (n). Notice radial trabeculae (rt). Bar: 0.33 µm. K. and L. Transverse sections of the nucleus (n) surrounded by radial trabeculae (rt) and the manchette (mt) Bar: 0.27 µm. In all figures: Acrosome cortex (co), acrosome medulla (me), epinuclear electron-lucent region (et), manchette (mt), microtubule embedded in dense material (*), nuclear shoulders (ns), plasma membrane (pm), perforatorium (p), subacrosomal clear zone (cz), subacrosomal cone (sc).
The nuclei of early spermatids present loose chromatin that is gradually compacted. During chromatin condensation, thick fibers are formed and twisted in a spiral arrangement then fused into a honeycomb arrange ment of lamellae (Figs. 2E, 2F, 2G). A microtubular structure, called the manchette, wraps helically around the nucleus in the beginning, straitening to a longitudinal arrangement with further nuclear condensation (Figs. 2F, 2G, 3I, 3J, 3K). These microtubules also surround the acrosomal complex (Figs. 3E, 3G). Completing its condensation, the nucleus becomes homogeneously electron dense (Figs. 3C, 3H, 3I, 3J, 3K, 3L), arched and conical in its anterior portion (Figs. 3A, 3B, 3C), where it is inserted in the subacrosomal cone (Fig. 3C). The nucleus is strongly fluorescent due to the specificity of the DAPI reaction, while the other structures fluoresce lightly, probably due to the fixative used (Fig. 3B). The transition between the conical and the cylindrical portion, called the nuclear shoulders, is abrupt and marks the posterior limit of the acrosome (Fig. 3C). A layer of radial trabeculae is observed between the nucleus and the microtubules of the manchette at the nuclear shoulders (Figs. 3I, 3J, 3K).
At the nuclear base, a deposit of electron dense material called the pericentriolar layer can be found (Figs. 4A, 4B, 4C). This region is considered the "spermatozoon neck" and consists of two centrioles, a proximal and a distal one, wrapped in the electron dense pericentriolar material (Figs. 2A, 2D, 4A, 4B, 4C). Nine triplets of microtubules constitute each centriole, associated to nine peripheral fibers and a central pair of microtubules, one of which has a dense fiber associated to it in the distal centriole (Figs. 4A, 4E). The distal centriole is short and maintains an intermediate position, just above the axoneme (Figs. 4A, 4B, 4C, 4D). These are surrounded by rings of rounded mitochondria, which alternate with dense bodies (Figs. 4C, 4D, 4E, 4F). Dense bodies develop between the mitochondria in the midpiece (Figs. 4D, 4E), forming inter-mitochondrial rings (Fig. 4D). The midpiece is limited by a dense ring called the annulus (Figs. 4A, 4B, 4C), which can be identified already in early spermatids (Figs. 2A, 2D). The initial piece of the flagellum is formed by an axoneme constituted by nine microtubule doublets, a central pair and peripheral dense fibers, associated to each of the nine pairs of microtubules (Figs. 4E, 4F). The principal piece of the flagellum consists in the axoneme (9+2), and vestigial peripheral dense fibers associated to pairs 3 and 8, all surrounded by the fibrous sheath and then the plasma membrane (Fig. 4G). The fibrous sheath is formed by regular individual rings, observed, in longitudinal sections, as two columns (Figs. 4D, 4F, 4G). The end piece of the flagellum is narrower and consists only of the axoneme, with peripheral dense fibers associated only to the 3rd and 8th pairs and covered by the plasma membrane (Fig. 4H). The peripheral dense fibers diminish in diameter along the length the of axoneme, and remain attached only to pairs 3 and 8 of axoneme up to the flagellar end piece (Figs. 4E, 4F, 4G, 4H).
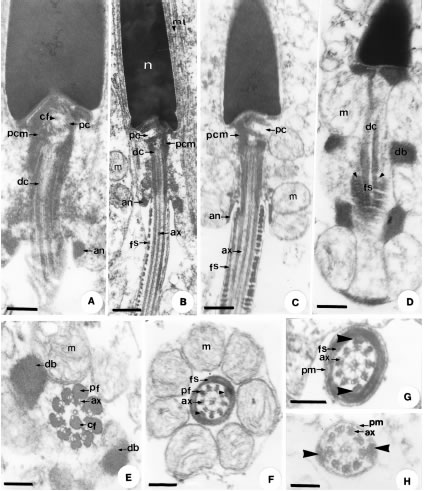
Figure 4. Transmission electron microscopy. A. - D. Longitudinal sections of the midpiece and flagellum regions. Notice the formation of structures such as the centriole pair, the aggregation of the pericentriolar material, the differentiation of the annulus, mitochondria and the dense bodies. Bar: 0.23 µm. E. Transverse section of the midpiece in the initial portion of the axoneme, with peripheral fibers associated to the doublets and one of the central microtubules. Bar: 0.10 µm. F. Transverse section of the end of the midpiece, where the axoneme presents vestigial peripheral fibers binding to the 3rd and 8th doublets and to the fibrous sheath (arrow head). Bar: 0.11 µm. G. - H. Transverse sections of the flagellum, in its principal piece (fig. G), formed by a 9+2 axoneme, connected to the fibrous sheath by the 3rd and 8th doublets (arrow head) and surrounded by the plasma membrane. The fibrous sheath begins to disappear (fig. H) in the end piece where the axoneme, still with the 9+2 pattern, retains dense fibers only on the 3rd and 8th doublets, connecting them to the plasma membrane. G. Bar: 0.10 µm, H. Bar: 0.08 µm. In all figures: Annulus (an), axoneme (ax), central singlet fiber (cf), dense bodies (db), distal centriole (dc), fibrous sheath (fs), mitochondria (m), pericentriolar material (pcm), peripheral dense fibers (pf), plasma membrane (pm), proximal centriole (pc).
Discussion
Among the structures found during spermiogenesis of T. itambere, some are shared with other vertebrate groups. Other characteristics are exclusive of the Squamata. The close association between Sertoli cells and spermatozoa observed in T. itambere presents a curious similarity with the formation of loose cysts in the seminiferous tubules, as in Aves (Góes and Dolder, 2002) e Amphibians (Taboga and Dolder, 1998; Báo et al., 1991).
The acrosome is an organelle rich in enzymes that participate in the penetration of the spermatozoon in the ovule (Baccetti and Afzelius, 1976). In Reptiles, the spermatids and spermatozoa have a considerable variation in the size, form, complexity and degree of compartmentalization. The compartmentalization of the acrosome could be an adaptation to facilitate the sequential release of acrosomal enzymes (Talbot, 1991). The presence of acrosomal layers covering the nuclear tip is a common feature in Squamata. According to Cruz-Landim and Cruz-Höfling (1977), the lizard Tropidurus torquatus has a homogeneous electron-dense acrosome but this does not occur in T. itambere. The presence of two distinct layers may indicate variations according to the degree of cell maturation. Similar to the acrosome of T. itambere are those of Phrynosomatidae (Scheltinga et al., 2000), Polychrotidae (Scheltinga et al., 2001) and Chamaleonidae (Al-Hajj et al., 1987; Dehlawi et al., 1992). Within the Tropiduridae family, acrosome and subacrosomal cone are always circular in transverse sections (Furieri, 1974; Teixeira et al., 1999) and can present an oval transverse section at the tip, as in other Iguania (Scheltinga et al., 2000; 2001) and Agamidae (Oliver et al., 1996). The epinuclear electron-lucent zone is absent in Scincidae (Jamieson and Scheltinga, 1993) and Gekkonidae (Jamieson et al., 1996). For the Squamata, in general, it is always present, varying from poorly developed, as in all Iguania (Oliver et al., 1996; Scheltinga et al., 2000; 2001) to very well developed as in Pygopodidae (Harding et al., 1995; Jamieson et al., 1996).
The origin of the perforatorium, during spermiogenesis, is unknown, but this structure has been described as being made up of a fibrous material, probably a cytoskeletal fibril (Baccetti and Afzelius, 1976). In terrestrial animals, the perforatorium consists of actin filaments which, during the acrosomal reaction, undergo conformational changes (Baccetti, 1986; Shiroya et al., 1986). In invertebrates, it is important for sperm penetration into the ovum (Baccetti et al., 1980), while in some birds it does not occur and therefore penetration cannot be attributed exclusively to the perforatorium. Therefore, Baccetti et al. (1980) suggested that it only supports the acrosome. In mammals, actin occurs only as a layer in the developing spermatids, where it contributes to nuclear and acrosome elongation, disappearing before final maturation (Baccetti et al., 1980; Baccetti, 1986). In some birds (Nagano, 1962; Humphreys, 1975) and reptiles (Del Conte, 1976), the granule found in the pro-acrosomal vesicle, in contact with the nucleus, has been pointed out as the origin of the perforatorium. According to Del Conte (1976) the granule may be produced by the interaction between the pro acrosomal vesicle and the nucleus. In Sphenodontes (Healy and Jamieson, 1992), Crocodilians (Saita et al., 1987; Jamieson et al., 1997), Chelonia (Sprando and Russel, 1988), Amphibians (Báo et al., 1991) and some Birds (Sprando and Russel, 1988) the perforatorium is located in the interior of intranuclear canals. In some Iguania, it enters the subacrosomal cone's apex below the perforatorium where an electron dense plate occurs, known as the perforatorial base plate (Scheltinga et al., 2000; 2001). However this base does not exist in T. itambere and other Tropidurus lizards (Teixeira et al., 1999; Vieira et al., 2001). This is one of the few differences between this species and the Iguanidae.
The acrosomal vesicle is derived from vesicles produced by both the endoplasmic reticulum and the Golgi complex, as was verified by Carcupino et al. (1989). This clearly seems to be the case in spermatids of T. itambere, considering the large amount of vacuoles of the aggregated endoplasmic reticulum, which could deliver their contents to the Golgi complex.
The great electron density of the nucleus, resulting from the extreme chromatin condensation, favors mobility and protects the genome against physical and chemical alterations during transport and storage (Krause, 1996). This elongated shape is established during spermiogenesis by the manchette (Soley, 1994) and by the degree of DNA and protein aggregation (Fawcett et al., 1971). All lizard spermatozoa are slender, as in T. itambere, except in Eugongulus, Scincidae (Jamieson and Scheltinga, 1993) that possess a larger diameter. Little has been said of the radial projections observed in the region described as the nuclear shoulders, observed in T. itambere and some Iguania (Al-Hajj et al., 1987; Vieira et al., 2001). Radial trabeculae are frequent in spermatids and spermatozoa of T. itambere. Cruz-Höfling and Cruz-Landim (1978) mentioned radial trabeculae of the nuclear envelope, but this aspect was not well discussed. We believe that these structures most likely establish connection between the microtubules of the manchette and the nucleus as defined by Butler and Gabri (1984) and Al-Hajj et al. (1987).
The centriole serves as a pattern for axoneme formation and this process is similar for all Squamata (Al-Hajj et al., 1987; Phillips and Asa, 1993). Nine peripheral dense fibers follow parallel to the nine doublets, as well as a single dense fiber attached to one of the central pair of microtubules is characteristic of all Squamata. Another characteristic for Squamata is the presence of a short distal centriole. It does not extend along the entire midpiece, ending well above the annulus, within the layer of encircling mitochondria. In Sphenodontia and Chelonia the distal centriole is long (Healy and Jamieson, 1992; Jamieson and Healy, 1992; Oliver et al., 1996). In Reptiles, the Crocodilia differ from the lizards, by the presence of a thick, dense fiber around one of the pair of central microtubules of the axoneme and the distal centriole (Saita et al., 1987; Jamieson et al., 1997). Among the Squamata, the spermatozoa almost always present linear mitochondrial cristae, inter-mitochondrial dense bodies, a short and distal centriole, and the fibrous sheath beginning already in the midpiece. The peripheral fibers of the distal centriole and axoneme are characteristic for Reptiles (Jamieson et al., 1997). These peripheral fibers apparently lend extra motor force, contributing to bending movements (Hamilton and Fawcett, 1968). Another function attributed to these peripheral fibers is a control mechanism for sperm movement (Anderson and Personne, 1969). These peripheral fibers also stabilize, longitudinally, the rings that make up the fibrous sheath (Austin, 1965). The fibrous sheath has elastic properties that also suggest a role in spermatozoon mobility (Fawcett, 1970).
The annulus occurs in most Tetrapoda and is also found in many Invertebrate groups (Baccetti and Afzelius, 1976). It consists of a set of closely associated filamentous subunits, adjoined and firmly adhering to the plasma membrane. Its function is to avoid the dislocation of mitochondria from the midpiece during flagellar movement (Fawcett, 1970). Differently from T. itambere, in Chelonia and Sphenodontia the mitochondria are sub-spherical, with concentric cristae and an intra-mitochondrial dense body (Furieri, 1970; Healy and Jamieson, 1994). The dense bodies in T. itambere form rings that closely resemble those of the Iguania (Oliver et al., 1996; Teixeira et al., 1999; Scheltinga et al., 2000; 2001). Other Squamata present dispersed or helically arranged dense bodies (Jamieson, 1995; Oliver et al., 1996). The dense bodies are considered to originate from mitochondria and are homologous to the intra-mitochondrial dense bodies (Carcupino et al., 1989; Healy and Jamieson, 1992). The pattern of the axoneme microtubules in the flagellar end piece of lizards varies greatly, where it may maintain the typical 9+2 arrangement (Scheltinga et al., 2000, 2001) or disorganize this typical pattern, as in other Squamata (Jamieson et al., 1996).
In conclusion, we present here the first detailed ultrastructural description of spermatogenesis in lizards. Although the initial cells are very similar to those of mammals, information on this phase is important. The observation of different phases of early spermatids has clarified the development of the structures described for the acrosomal complex. The mature acrosome, the presence of an electron dense medulla and a clear cortex is clearly estabilished, which is contrary to the existing descriptions of the Tropiduridae family. In the terminal tail portion, the vestigial peripheral dense fibers continue, associated to the 3rd and 8th pairs of axoneme microtubules, which was not observed in Iguanidae. The similarities observed between T. itambere (Tropiduridae), Iguanidae and Agamidae contribute toward the confirmation of a close phylogenetic relationship between these three families. The above characteristics may be used as evidences that these families should really be separated, as has been recently established (Frost, 1992).
Acknowledgements
The research was supported by the FAPESP (Fundação de Apoio à Pesquisa do Estado de São Paulo, File # 01/06744-4). The animals were collected with authorization of the MMA/IBAMA (Ministério do Meio Ambiente / Instituto Brasileiro do Meio Ambiente, File # 02027.003521/02-17).
References
1. Al-Hajj H, Janakat S, Mahmoud F (1987). Electron microscopic study of sperm head differentiation in the lizard Agama stellio. Can J Zool. 65: 2959-2968. [ Links ]
2. Amat F, Llorente GA, Carretero MA (2000). Reproductive cycle of the sand lizard (Lacerta agilis) in its southwestern range. Amphibia-Reptilia 21: 463-476. [ Links ]
3. Anderson WA, Personne P (1969). Structure and histochemistry of basal body derivative, neck and axoneme of spermatozoa of Helix aspersa. J Microsc. 8: 87-96. [ Links ]
4. Austin CR (1965). Fine structure of the snake sperm tail. J Ultrastruc Res. 12: 452-462. [ Links ]
5. Baccetti B (1986). Evolutionary trends in sperm structure. Comp Biochem Physiol. 85: 29-36. [ Links ]
6. Baccetti B, Afzelius BA (1976). The biology of the sperm cell. Monogr Dev Biol. 10: 1-124. [ Links ]
7. Baccetti B, Bigliardi E, Burrini AG (1980). The morphogenesis of the vertebrate perforatorium. J Ultrastruct Res. 71: 272-287. [ Links ]
8. Bao SN, Dalton GC, Oliveira SF (1991). Spermiogenesis in Odontophrynus cultripes (Amphibia, Anura, Leptodactylidae): ultrastructural and cytochemical studies of proteins using E-PTA. J Morphol. 207: 303-314. [ Links ]
9. Butler RD, Gabri MS (1984). Structure and development of the sperm head in the lizard Podarcis (=Lacerta) taurica. J Ultrastruct Res. 88: 261-274. [ Links ]
10. Carcupino M, Corso G, Pala M (1989). Spermiogenesis in Chalcides ocellatus tiligugu (Gmelin) (Squamata: Scincidae): an electron microscope study. Boll Zool 56: 119-124. [ Links ]
11. Clark AW (1967). Some aspects of spermiogenesis in a lizard. Amer J Anat. 121: 369-400. [ Links ]
12. Cruz MVS, Mendez de La Cruz FR, Parragamez L (1994). Spermatogenesis in the lizard Sceloporus mucronatus (Reptilia, Phrynosomatidae). Rev Biol Trop. 42: 289-296. [ Links ]
13. Cruz-Höfling MA, Cruz-Landim C (1978). The fine structure of nuclei during spermiogenesis in the lizard Tropidurus torquatus (Lacertilia). Cytologia 43: 61-68. [ Links ]
14. Cruz-Landim C, Cruz-Höfling MA (1977). Electron microscope study of lizard spermiogenesis in Tropidurus torquatus (Lacertilia). Caryologia 30: 151-162. [ Links ]
15. Dehlawi GY, Ismail MF (1990). Studies on the ultrastructure of the spermiogenesis of Saudian Reptiles 1 _ The sperm head differentiation in Uromatyx philbyi. Proc Zool Soc Arab Rep Egyp. 21: 79-89. [ Links ]
16. Dehlawi GY, Ismail MF, Hamdi SA, Jamjoom MB (1992). Ultrastructure of spermiogenesis of Saudian Reptiles 6 _ The sperm head differentiation in Agama adramitana. Arch Androl. 28: 223-234. [ Links ]
17. Del Conte E (1976). The subacrosomal granule and its evolution during spermiogenesis in a lizard. Cell Tiss Res. 171: 483-498. [ Links ]
18. Fawcett DW (1970). A comparative view of sperm ultrastructure. Biol Reprod (Suppl.) 2: 90-127. [ Links ]
19. Fawcett DW, Anderson WA, Phillips DM (1971). Morphogenetic factors influencing the shape of the sperm head. Develop Biol. 26: 220-251. [ Links ]
20. Ferreira A, Dolder H (2002). Ultrastructural spermiogenesis of Iguana iguana (Reptilia: Sauria: Iguanidae). Eur J Morphol. 40: 89-99. [ Links ]
21. Frost DR (1992). Phylogenetic analysis and taxonomy of the Tropidurus groups of lizards (Iguania: Tropiduridae). Am Mus Novitates 3033: 1-68. [ Links ]
22. Furieri P (1970). Sperm morphology in some reptiles: Squamata and Chelonia. In: Comparative spermatology (Procceding of the International Symposium). B. Baccetti, Ed. Accademia Nazionale dei Lincei, Rome, pp. 115-143. [ Links ]
23. Furieri P (1974). Spermi e spermatogenesi in alcuni iguanidi argentini. Riv Biol. 67: 233-279. [ Links ]
24. Góes RM, Dolder H (2002). Cytological steps during spermiogenesis in the house sparrow (Passer domesticus, Linnaeus). Tissue Cell 34: 265-274. [ Links ]
25. Hamilton DW, Fawcett DW (1968). Unusual features of the neck and middle-piece of snake spermatozoa. J Ultrastruct Res. 23: 81-97. [ Links ]
26. Harding HR, Aplin KP, Mazur M (1995). Ultrastructure of spermatozoa of Australian blindsnakes, Ramphotyphlops spp. (Typhlopidae, Squamata): first observations on the mature spermatozoon of Scolecophidian snakes. In: Advances in Spermatozoal Phylogeny and Taxonomy. BGM. Jamieson, J. Ausio, JL. Justine, Eds. Mém. Mus. Natn. Hist. Nat. 166: 385-396. [ Links ]
27. Healy JM, Jamieson BGM (1992). Ultrastructure of spermatozoon of the tuatara (Sphenodon punctatus) and is relevance to the relationships of the Sphenodontida. Phil Trans R Soc Lond B. 335: 193-205. [ Links ]
28. Healy JM, Jamieson BGM (1994). The ultrastructure of spermatogenesis and epididymal spermatozoa of the tuatara Sphenodon punctatus (Sphenodontida: Amniota). Phil Trans R Soc Lond B. 344: 187-199. [ Links ]
29. Humphreys PN (1975). The differentiation of the acrosome in the spermatid of the budgerigar (Melopsittacus undulatus). Cell Tissue Res. 156: 411-416. [ Links ]
30. Ibarguengoytia NR, Cussac VE (1999). Male response to low frequency of female reproduction in the viviparous lizard Liolaemus (Tropiduridae). Herpetol J. 9: 111-117. [ Links ]
31. Jamieson BGM (1995). The ultrastructure of spermatozoa of the Squamata (Reptilia) with phylogenetic considerations. In: Advances in spermatozoal phylogeny and Taxonomy. BGM. Jamieson, J. Ausio, JL. Justine, Eds. Édition du muséum national d'histoire naturelle, Paris, pp. 395-383. [ Links ]
32. Jamieson BGM, Healy JM (1992). The phylogenetic position of the tuatara, Sphenodon (Sphenodontida: Aminiota), as indicated by cladistic analysis of the ultrastructure of spermatozoa. Phil Trans R Soc Lond B. 335: 207-219. [ Links ]
33. Jamieson BGM, Scheltinga DM (1993). The ultrastructure of spermatozoa of Nangura spinosa (Scincidae, Reptilia). Mem Queensland Mus. 34: 169-179. [ Links ]
34. Jamieson BGM, Oliver SC, Scheltinga DM (1996). The ultrastructure of the spermatozoa of Squamata. I. Scincidae, Gekkonidae and Pygopodidae (Reptilia). Acta Zool (Stockholm) 77: 85-100. [ Links ]
35. Jamieson BGM, Scheltinga DM, Tucker AD (1997). The ultrastructure of spermatozoa of the Australian freshwater crocodile, Crocodylus johnstoni Krefft, 1873 (Crocodylidae, Reptilia). J Submicr Cytol Pathol. 29: 265-274. [ Links ]
36. Krause WJ (1996). Meiosis and male reproductive organs. In: Essentials of Human Histology. WJ. Krause, Ed. Little, Brown and Company, New York, pp. 331-352. [ Links ]
37. Martinage A, Depeiges A, Wouters D, Morel L, Sautiere P (1996). Spermatogenesis of the lizard Lacerta vivipara: Histological studies and amino acid sequence of protamine lacertine 1. Comp. Ren. Acad. Scien. III _ Scien. Vie-Life Scien. 319: 511-516. [ Links ]
38. Nagano T (1962). Observations on the fine structure of the developing spermatids in the domestic chicken. J Cell Biol. 14: 193-205. [ Links ]
39. Oliver SC, Jamieson BGM, Scheltinga DM (1996). The ultrastructure of spermatozoa of Squamata. II. Agamidae, Varanidae, Colubridae, Elapidae and Boidae (Reptilia). Herpetologica 52: 216-241. [ Links ]
40. Phillips DM, Asa CS (1993). Strategies for formation of the midpiece. In: Comparative spermatology 20 years after. B. Baccetti, Ed. Raven Press, New York, pp. 997-1000. [ Links ]
41. Rodrigues MT (1987). Sistemática, ecologia e zoogeografia dos Tropidurus do grupo Torquatus ao sul do rio Amazonas (Sauria: Iguanidae). Arq Zool SP. 31: 105-230. [ Links ]
42. Saita A, Comazzi M, Perrota E (1987). Electron microscope study of spermiogenesis in Caimam crocodylus L. Boll Zool. 4: 307-318. [ Links ]
43. Saita A, Comazzi M, Perrota E (1988). New data at the E. M. on the spermiogenesis of Iguana delicatissima (Laurent) involving comparative significance. Acta Embryol Morphol Exper. 9: 105-114. [ Links ]
44. Scheltinga DM, Jamieson BGM, Trauth SE, Mcallister CT (2000). Morphology of the spermatozoa of the Iguanian lizards Uta stanburiana and Urosaurus ornatus (Squamata, Phrynosomatidae). J Submicrosc Cytol Pathol. 32: 261-271. [ Links ]
45. Scheltinga DM, Jamieson BGM, Espinoza RE, Orrel KS (2001). Descriptions of the mature spermatozoa of the lizards Crotaphytus bicinctores, Gambelia wislizenii (Crotaphytidae), and Anolis carolinensis (Polychrotidae) (Reptilia, Squamata, Iguania). J Morphol. 247: 160-171. [ Links ]
46. Shiroya Y, Hosoya H, Mabuchi I, Sakai YT (1986). Actin filament bundle in the acrosome of Abalone spermatozoa. J Exp Zoo. 239: 105-115. [ Links ]
47. Soley JT (1994). Centriole development and formation of the flagellum during spermiogenesis in the ostrich (Struthio camelus). J Anat. 185: 301-313. [ Links ]
48. Sprando RL, Russel LD (1988). Spermiogenesis in red-ear turtle (Pseudemys scripta) and the domestic fowl (Gallus domesticus): a study of cytoplasmic elimination. J Morphol. 198: 95-118. [ Links ]
49. Taboga SR, Dolder H (1998). Spermiogenesis of Scinax ranki (Amphibia, Anura, Hylidae): nuclear compactation and associated cytoplasmic events. Braz J Morphol Sci. 15: 157-163. [ Links ]
50. Talbot P (1991). Compartimentalization in the acrosome. In: Comparative spermatology 20 years after. B. Baccetti, Ed. Raven Press, New York, pp. 255-258. [ Links ]
51. Teixeira RD, Vieira GHC, Colli GR, Báo SN (1999). Ultrastructural study of spermatozoa of the neotropical lizards, Tropidurus semiaeniatus and Tropidurus torquatus (Squamata: Tropiduridae). Tissue Cell 31: 308-317. [ Links ]
52. Van Sluys M (1993). The reproductive cycle of Tropidurus itambere (Sauria: Tropiduridae) in Southeastern Brazil. J Herpetol. 27: 28-32. [ Links ]
53. Vieira GHC, Wiederhecker HC, Colli GR, Báo SN (2001). Spermiogenesis and testicular cycle of the lizard Tropidurus torquatus (Squamata, Tropiduridae) in the Cerrado of central Brazil. Amphibia-Reptilia 22: 217-233. [ Links ]
Received on April 7, 2003.
Accepted on August 11, 2003.














