Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.27 n.3 Mendoza ago./dic. 2003
Ofelia Acosta de Pérez1, Laura Leiva de Vila2, María Elisa Peichoto2, Silvana Maruñak3, Raquel Ruíz3, Pamela Teibler3, Carolina Gay2 and Laura Rey4
1. CONICET, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste (UNNE). Sargento Cabral 2139, (3400) Corrientes, Argentina.
2. Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste (UNNE). Corrientes, Argentina.
3. Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste (UNNE). Corrientes, Argentina.
4. Serpentario del Zoológico de la Ciudad de Corrientes. Corrientes, Argentina.
Address correspondence to: Dra. Ofelia Acosta de Pérez. Cátedra de Farmacología, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste (UNNE). Sargento Cabral 2139, (3400) Corrientes, ARGENTINA. Tel/Fax: (+54-3783) 425753 int. 146; E-mail: patmed@vet.unne.edu.ar
Abstract: Philodryas olfersii is found in South America, from Amazonas to Patagonia. It is important to characterize the venom of P. olfersii, who inhabits the North-East region of Argentina, since snake venoms are known to exhibit considerable variability in composition and biological activities. In this work, mice weighing 18-20 g (n = 4 for each experimental group) were used. For the edematogenic activity mice were injected s.c. in the right foot pad with 50 ml of solutions containing different amounts of venom, whereas the left foot pad was injected with 50 ml of PBS. Two hours after injection mice were killed by cervical dislocation and both feet were cut off and weighed individually. For the myotoxic activity mice were injected i.m. with 100 ml of solutions containing 40 mg of venom. Blood samples were extracted after 1, 3, 6, 8, 10, 12, 14, 16 and 24 h of venom injection to determinate serum CPK activity and mice were sacrificed at the same time intervals to obtain the inoculated gastrocnemius muscle. They were fixed with Bouin solution and stained with Hematoxylin-Eosin. Results showed that P. olfersii venom exhibits a high edematogenic activity (MED = 0.31 mg) and a moderate myotoxic activity. Myonecrosis reached its highest level after 12 h of venom injection as shown by plasmatic CPK levels (5,401 ± 330 IU/l) and microscopic assay. It demonstrates the potential toxicity of the venom of P. olfersii, who inhabits the North-East region of Argentina. It also reinforces the original warning concerning the potential danger of bites by colubrids.
Keywords: Philodryas olfersii. Edematogenic activity. Myotoxic activity.
Introduction
Reports on toxic saliva in colubrid snakes are few and the chances of being bitten by colubrid snakes are rare, because of its non-aggressive behavior and the anatomical disavantage of having fangs situated behind its other teeth. Nevertheless, some species have caused severe reactions in humans. Paralysis, respiratory failure, hemorrhage and death have been observed. While many reports are found on venom constituents of snakes belonging to the families Elapidae, Hydrophidae, Crotalidae and Viperidae, because of the small quantity of secretion of the Duvernoy's gland, very little has been done to elucidate the composition and biological activities of colubrid venoms (Assakura et al., 1992, 1994). It is worthwhile to point out that non-clotting blood and hemorrhage, usually associated with viperid envenomations, are the most striking clinical manifestations of colubrid envenomations (Vest, 1978; Visser and Chapman, 1978; Silva and Buononato, 1983/84).
Among the snakes belonging to the Colubridae family, the genus Philodryas are widespread all over South America and are considered as not venomous. However, they have a Duvernoy's gland, whose secretion possesses a toxicity such as to bring about serious lesions (Assakura et al., 1992, 1994).
There have been reports of bites in humans by various Philodryas species: P. aestivus (Fowler and Salomão, 1994), P. baroni (Kuch and Jesberger, 1993), P. olfersii (Nickerson and Henderson, 1976; Silva and Buononato, 1983/84; Fowler and Salomão, 1994) and P. patagoniensis (Fowler and Salomão, 1994; Nishioka and Silveira, 1994). However, the most studied species so far is Philodryas olfersii (green snake) .
Philodryas olfersii, a Colubridae-Xenodontinae, is an opistoglyphous snake with a well-developed Duvernoy's gland connected with a grooved tooth. This snake is found in South America, from Amazonas to Patagonia (Assakura et al., 1992, 1994).
Accidents with Philodryas olfersii, although rare and not lethal, are usually painful, followed by edema and hemorrhage of the limb (Martins, Ph. D. Thesis, São Paulo, 1916; Nickerson and Henderson, 1976; Silva and Buononato, 1983/84). Most of the manifestations at the bite site could be caused by both, the mechanical trauma of the bite and the local activity of toxins who are present in the venom (Ribeiro et al., 1999).
The venom of Philodryas olfersii has high hemorrhagic, edema-inducing and fibrin(ogen)olytic activities. However, it is devoid of thrombin-like, procoagulant, phospholipase A2 and platelet aggregating enzymes (Assakura et al., 1992).
Local symptoms can be misinterpreted as envenoming by Bothrops species (yarará) (Cardoso et al., 1993; Ribeiro and Jorge, 1997), who are present in the same region (Campbell and Lamar, 1989), what may result in inappropriate antiserum treatment (Nishioka and Silveira, 1994).
Snake venoms are known to exhibit considerable variability in composition and biological activities, which may due to inherent, but genetically variable, characteristics of specific protein synthesis or to exogenous factors such as habitat conditions, climate, age, and feeding habits (Kuch et al., 1996; Cavinato et al., 1998; World Health Organization, 1971; Gutiérrez et al., 1980). For that reason, it is important to characterize the Duvernoy's gland secretion of Philodryas olfersii, who inhabits the North-East region of Argentina. The present work will concentrate on the necrotizing and edema-forming effects of Philodryas olfersii Duvernoy's gland secretion.
Material and Methods
Material
Duvernoy's gland secretion of Philodryas olfersii was obtained from adult snakes measuring 90-120 cm and kept in the local Zoo (Corrientes, Argentina). The snakes were milked by introducing a 100 ml micropipet over each fang following the procedure of Ferlan et al. (1983). Crude venom was lyophilized; after that, it was kept frozen at _20ºC. When required, the venom was diluted with phosphate buffered saline solution, pH 7.2. The small amount of insoluble material was centrifuged and the clear supernatant was applied for studies.
The protein concentration of venom solutions was determined by measuring the absorbance at 280 nm in a 1 cm cell, based upon the assumption that the absorbance of 1 mg/ml of crude venom was 1.183.
Solutions containing the venom diluted in phosphate buffered saline solution (pH 7.2) were used for inoculating male white mice belonging to the line CF1 and weighing 18 to 20 g.
Methods
Edema-forming activity
Method of Yamakawa et al. (1976) was used. Five groups of four mice were injected s.c. in the right foot pad with 50 ml of solutions containing different amounts of venom (from 0.05 to 5 mg), whereas the left foot pad was injected with 50 ml of phosphate buffered saline solution (pH 7.2). Mice were anesthetized with i.p. injection of cloral hydrate and killed by cervical dislocation 1 h after injection and both feet were cut off and weighed individually. Edema was expressed as the percentage increase in weight of the right foot compared to that of the left one. The minimum edema dose (MED) was defined as the least quantity of venom causing 30% increase in the weight, compared to the control.
Myotoxic activity
Groups of 4 mice (animals for which the LD50 had previously been reported by Assakura et al. (1992) to be 2.8 mg/kg) were injected i.m. in the right gastrocne mius with 40 mg of the whole Duvernoy's secretion dissolved in 0.1 ml of phosphate buffered saline solution, pH 7.2. Four mice were used as control samples, receiving each of them 0.1 ml of phosphate buffered saline solution (pH 7.2). After 1, 3, 6, 8, 10, 12, 14, 16 and 24 h of venom injection, mice were anesthetized with cloral hydrate i.p. 300 mg/kg to collect blood samples without using anticoagulant. Serum was obtained to analyze the activity of creatinphosphokinase (CPK) with the kinetic method UV (GT laboratory) based on the measurement of the creatinine formed in the reaction ADP/phosphocreatine. Creatinphosphokinase activity was expressed in units per liter. In order to have a histological assessment of miotoxicity, mice were sacrificed by cervical dislocation 1, 3, 6, 8; 10; 12; 14; 16 and 24 hours after venom injection and samples of injected muscle were taken and fixed with Bouin solution for 24-48 h. Thereafter, the muscle was dehydrated in a graded alcohol series and embedded in paraffin. Sections 10 mm thick were stained with Hematoxylin-Eosin (HE). Control muscles were processed in an identical manner.
Necrosis was classified according to the method of Homma and Tu (1971), based on the morphology of the necrotic fibers. The myolitic type was characterized by fibrilar material, alterning with clear areas. In the coagulative necrosis type, the fibers acquired a hyaline appearance and its distribution was homogeneous.
Statistical analysis
Statistical evaluation was performed using Statistics 4.5 (Statsoft Inc. USA) on a Pentium personal computer. Comparisons between groups were made using a Student's test, using a t critical calculated with Bon Ferroni corrections. The CPK enzyme was evaluated by the variance analysis. P < 0.05 was considered statistically significant.
Results
Edema-forming activity
The Duvernoy's gland secretion of Philodryas olfersii exhibited intense edematogenic activity when tested by the foot-pad assay (four mice per dose were used, n = 4). The percentage increases in weight of the right foot compared to that of the left were evaluated 1 h after venom injection and were proportional to the amount of venom injected; the obtained linear relationship (r = 0.9371) let us to determine the MED. Compared to mouse foot pads injected with phosphate buffered saline solution, a 30% increase in the weight was produced by injecting 0.31 mg of the venom.
Myotoxic activity
After i.m. injection, Philodryas olfersii Duvernoy's gland secretion induced a late increase in serum creatinphosphokinase (Fig. 1), which is a specific marker for muscle damage. At a dose of 40 mg/animal, the CPK levels after 1 h and 3 h were low. Maximum levels were observed after 12 h, and decreased afterward. CPK levels after 24 h were similar to those obtained with control samples.

Figure 1. Changes in serum creatinphosphokinase (CPK) levels of mice after i.m. injection of 40 µg Philodryas olfersii Duvernoy's gland secretion. At various time intervals, mice were bled and serum CPK levels determined. CPK activity is expressed in international units/l. Results are presented as means ± SE (n = 4).
Histological observations in samples of gastrocnemius muscle obtained 1, 3, 6, 8, 10, 12, 14, 16 and 24 h after venom injection confirmed myotoxicity and edema-forming activity. Control sample retained a normal appearance (Fig. 2. A), whereas those samples of gastrocnemius muscle inoculated with 40 mg of P. olfersii venom showed intense damage characterized by edema, hemorrhage, polymorphonuclear neutrophil inflammatory infiltrate and myonecrosis (Fig. 2.B-C-D-E). Sixty minutes after venom injection, histological cuttings of gastrocnemius muscle showed interfibrilar hemorrhage and normal muscular fibers (Fig. 2.B). Three hours later, necrotic muscular fibers, polymorphonuclear neutrophil inflammatory infiltrate, extensive hemorrhagic effects and interfibrilar edema were observed (Fig. 2.C). Results obtained 6 h after venom injection were not significantly different from those of 3 h later. Twelve hours later, edema, hemorrhage, polymorphonuclear neutrophil inflammatory infiltrate and necrotic muscular fibers were observed. Myonecrosis belonging to myolitic type reached its highest level after 12 h of venom injection (Fig. 2.D). Twenty four hours later, abundant amount of rests of celular membranes, resulting of the phagocytic process carried out by neutrophils, was observed. Blood red cells remained in the interstice, sorrounded by edema (Fig. 2.E). CPK release, observed in in vivo assays, was coincident with results obtained from these preparations.


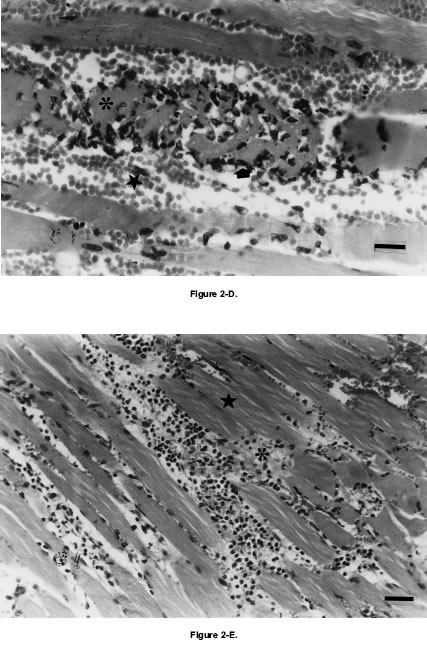
Figure 2. A- Longitudinal section of control mouse gastrocnemius muscle. Bar represents 20 µm. B-; C-; D-; E- Paraffin histological section of mouse gastrocnemius muscle after i.m. injection of 40 µg of Philodryas olfersii Duvernoy's gland secretion. B- After 60 min: note interfibrilar hemorrhage and normal muscular fibers (asterisk). Bar represents 20 µm. C- After 3 h: note necrosis of muscular fibers (asterisk), polymorphonuclear neutrophil inflammatory infiltrate (arrow) and hemorrhage (star). Bar represents 20 µm. D- After 12 h: note myolitic necrosis (asterisk), polymorphonuclear neutrophil inflammatory infiltrate (arrow) and hemorrhage (star). Bar represents 20 µm. E- After 24 h: note rests of necrotic fibers (asterisk) sorrounded by neutrophils and hemorrhage, and normal muscular fibers (star). Bar represents 45 µm. (Hematoxylin-Eosin).
Discussion
The purpose of this work was to characterize the necrotizing and edema-forming effects of the Duvernoy's gland secretion of Philodryas olfersii, who inhabits the North-East region of Argentina, and however, it is poorly studied in this country. New information about this venom could help to improve the interpretation of the lesions caused by the bite of this colubrid snake, being it is very important to prevent people from receiving the incorrect treatment, which could have harmful effects on the patient's health.
Swelling and edema are often the paramount early features of snake venom poisoning at the affected part of the victims (Reid, 1968). Duvernoy's gland secretion of Philodryas olfersii, who inhabits the North-East region of Argentina, showed a value of edema-producing activity similar to that reported by Assakura et al., 1992 (0.25 mg). This edema-forming activity is much higher than the edema caused by venom of Bothrops species from Argentina: B. jararaca, B. jararacussu,B. neuwiedii and B. alternatus, who present values of MED of 0.85 mg, 1.5 mg, 2.05 mg and 4.00 mg, respectively (Acosta et al., 1998).
Compared to hemorrhagic activity, little is known about the importance of myotoxicity in the mode of action of Duvernoy's gland secretions from colubrid snakes (Weinstein and Kardong, 1994). Prado-Franceschi et al. (1996, 1998) studied the creatinphosphokinase (CPK) release after 3 h and 5 h of the injection in gastrocnemius muscle of 20 mg and 40 mg of P. olfersii venom, and effects of whole Duvernoy's secretion on mouse phrenic nerve-diaphragm and chick biventer cervicis preparations, obtaining similar results to those described above for the secretion of P. olfersii coming from Argentina. Results of our investigation, about the effects of whole Duvernoy's secretion on mouse gastrocnemius muscle, clearly show that P. olfersii venom induces various morphological alterations which result in myonecrosis. CPK release in in vivo assays, studied until after 24 h of venom injection, is further evidence of a direct myolytic action of the whole Duvernoy's secretion, reaching maximum levels after 12 h of venom injection. The morphological appearance of small portions of muscle obtained 60 min after the in vivo administration of whole secretion was not significantly different from that of control tissues (injected with phosphate buffered saline solution). Similarly, levels of CPK released within 60 min were slightly higher than those obtained with control samples. Histological observations in samples of gastrocnemius muscle showed hemorrhage from 1 h of venom injection, increasing its intensity until reaching its maximum level between 6 and 12 h.
Accompaning to the hemorrhage, a polymorphonuclear neutrophil inflammatory infiltrate was observed. Maximum arrival of neutrophils also took place between 6 and 12 h. Edema was also observed, which was increasing progressively from 3 h of the venom injection. The latter was accompanied by necrosis of muscular fibers, mainly belonging to myolitic type, which reached maximum levels after 12 h of venom injection. At this time, maximum levels of creatinphosphokinase release was also observed, confirming the maximum level of muscular damage. After 24 h of venom injection, edema remained and muscular fibers were empty, with scarce blood white cells and abundant amount of dispersed celular membranes. This situation suggests us that the damaged field is in conditions of iniciating the process of muscular regeneration.
The light microscopy findings reveal little about the mechanism(s) of the myotoxicity involved here. The venom of Philodryas olfersii has high hemorrhagic and proteolytic activities. However, it is devoid of phospholipase A2 enzyme (Assakura et al., 1992). For that reason, we can presume that the late myonecrotic effect of the venom could be due to either the local ischemia resulting of the hemorrhagic action of the venom (detected in histological cuttings through blood red and white cells infiltration, being it already observed after 60 min of the i.m. injection of 40 mg of P. olfersii venom) and the action of proteolytic enzymes. In order to examine the relationship of necrotic activity to hemorrhagic and proteolytic activities in Philodryas olfersii venom, more morphological and biochemical studies are necessary to carry out. Further elucidation of the nature of venom components that induce necrosis obviously depends on the devising of a reliable method for quantitative assay of necrotic activity.
The low value obtained for the MED and the muscular damage observed in the fibers of injected gastrocnemius muscle, although the latter is later and less intense than that observed with Bothrops venoms (Gutiérrez and Lomonte, 1995), demonstrate the potential toxicity of the venom of P. olfersii, who inhabits the North-East region of Argentina. It also reinforces the original warning concerning the potential danger of bites by colubrids (Minton, 1978).
References
1. Acosta de Pérez OC, Koscinczuk P, Teibler P, Sánchez Negrette M, Ruíz R, Maruñak S, Bogarín G (1998). Actividades hemorrágica y edematizante y alteraciones histológicas en almohadilla plantar del ratón inducidas por venenos de serpientes de los géneros Bothrops y Crotalus de Argentina. Toxicon 36: 1165-1172. [ Links ]
2. Assakura MT, Reichl AP, Mandelbaum FR (1994). Isolation and characterization of five fibrin(ogen)olytic enzymes from the venom of Philodryas olfersii (green snake). Toxicon 32: 819-831. [ Links ]
3. Assakura MT, Salamão MG, Puorto G, Mandelbaum FR (1992). Hemorrhagic, fibrinogenolytic and edema forming activities of the venom of the colubrid snake Philodryas olfersii (green snake). Toxicon 30: 427-438. [ Links ]
4. Campbell JA, Lamar WW (1989). The venomous reptiles of Latin America. Canstock Publishing Associates, Ithaca. [ Links ]
5. Cardoso JLC, Fan HW, Franca FOS, Jorge MT, Leite RP, Nishioka SA, Avila A, Sano Martins IS, Tomy SC, Santoro ML, Chudzinski AM, Castro SCB, Kamiguti AS, Kelen EMA, Hirata MH, Mirandola RMS, Theakston RDG, Warrell DA (1993). Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 86: 315-325. [ Links ]
6. Cavinato RA, Remold H, Kipnis IL (1998). Purification and variability in thrombin-like activity of Bothrops atrox venom from different geographic regions. Toxicon 36: 257-267. [ Links ]
7. Ferlan I, Ferlan A, King T, Russel FE (1983). Preliminary studies on the venom of the colubrid snake Rhabdophis subminiatus (red-necked keelback). Toxicon 21: 570-574. [ Links ]
8. Fowler IR, Salomão M da G (1994). Activity patterns in the colubrid snake genus Philodryas and their relationship to reproduction and snakebite. Bull Chicago Herp Soc. 29: 229-232. [ Links ]
9. Gutiérrez JM, Chaves F, Bolaños R (1980). Comparative study of venoms of newborn and adult specimens of Bothrops asper. Rev Biol Trop. 228: 341-351. [ Links ]
10. Gutiérrez JM, Lomonte B (1995). Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon 33: 1405-1424. [ Links ]
11. Homma M, Tu AT (1971). Morphology of local tissue damage in experimental snake envenomation. Br J Exp Pathol. 52: 538-542. [ Links ]
12. Kuch U, Jesberger U (1993). Human envenomation from the bite of the South American colubrid snake species Philodryas baroni Berg, 1895. Snake 25: 63-65. [ Links ]
13. Kuch U, Mebs D, Gutiérrez JM, Freire A (1996). Biochemical and biological characterization of ecuadorian pitviper venoms (genera Bothriechis, Bothriopsis, Bothrops and Lachesis. Toxicon 34: 714-717. [ Links ]
14. Martins N (1916). Das opistoglyphas brasileiras e seu veneno. Colet Trab Instituto Butantan 427-496. [ Links ]
15. Minton SA (1978). Beware: nonpoisonous snakes. Nat Hist, NY 87: 56. [ Links ]
16. Nickerson MA, Henderson RW (1976). A case of envenomation by the South American colubrid, Philodryas olfersii. Herpetologica 32: 197-198. [ Links ]
17. Nishioka S de A, Silveira PVP (1994). Philodryas patagoniensis bite and local envenoming. Rev Inst Med Trop São Paulo 36: 279-281. [ Links ]
18. Prado-Franceschi J, Hyslop S, Cogo JC, Andrade AL, Assakura M, Cruz-Höfling MA, Rodrigues-Simioni L (1996). The effects of Duvernoy's gland secretion from xenodontine colubrid Philodryas olfersii on striated muscle and the neuromuscular junction: partial characterization of a neuromuscular fraction. Toxicon 34: 459-466. [ Links ]
19. Prado-Franceschi J, Hyslop S, Cogo JC, Andrade AL, Assakura M, Reichl A P, Cruz-Höfling MA, Rodrigues-Simioni L (1998). Characterization of a myotoxin from the Duvernoy's gland secretion of the xenodontine colubrid Philodryas olfersii (green snake): effects on muscle and the neuromuscular junction. Toxicon 36: 1407-1421. [ Links ]
20. Reid HA (1968). Symptomatology, pathology and treatment of land snake bite in India and Southeast Asia. In: Bücherl, W., Buckley, E., Deulofeu, V. (Eds.). Venomous Animals and Their Venoms, Vol. I, pp. 611-642. New York. Academic. [ Links ]
21. Ribeiro LA, Puorto G, Jorge MT (1999). Bites by the colubrid snake Philodryas olfersii: a clinical and epidemiological study of 43 cases. Toxicon 37: 943-948. [ Links ]
22. Ribeiro LA, Jorge MT (1997). Acidente por serpentes do género Bothrops: série de 3139 casos. Rev Soc Brasil Med Trop 30: 475-480. [ Links ]
23. Silva MV, Buononato MA (1983/84). Relato clínico de envenenamiento humano por Philodryas olfersii. Mem Inst Butantan 47/48: 121-126. [ Links ]
24. Vest DK (1978). Some effects and properties of Duvernoy's gland secretion from Hypsiglena torquata texana (Texas night snake). Toxicon 26: 417-419. [ Links ]
25. Visser J, Chapman DS (1978). In: Snakes and snake bites. Venomous Snakes and Management of Snake Bite in Southern Africa, pp. 61-131 (PURNELL, Mc. D., Ed.). Johannesburg. [ Links ]
26. Weinstein SA, Kardong KV (1994). Properties of Duvernoy's secretion from opistoglyphous and aglyphous colubrid snakes. Toxicon 32: 1161-1185. [ Links ]
27. World Health Organization (1971). Informe Técnico de Patrones Biológicos, Publicación Nº 37. Ginebra. [ Links ]
28. Yamakawa M, Nozaki M, Hokama Z (1976). Fractionation of Sakishima-Habu (Trimeresurus elegans) venom and lethal hemorrhagic, and edema forming activities of the fractions. In: Ohsaka, A.; Hayashi, K. and Sawai, Y (eds.). Animal, plant and microbial toxins, vol, 1, Biochemistry. Plenum Press, New York, p. 97-109. [ Links ]
Received on May 16, 2003.
Accepted on September 16, 2003.














