Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.28 n.1 Mendoza jan./abr. 2004
Tissue alterations in the Guinea pig lateral prostate following antiandrogen flutamide therapy
Renato Simões Cordeiro*, Wellerson Rodrigo Scarano*, Rejane Maira Góes**, And Sebastião Roberto Taboga**
* Department of Cell Biology, Institute of Biology, UNICAMP, CP 6109 - 13084-971. Campinas, SP, Brazil.
** Laboratory of Microscopy and Microanalysis, Department of Biology, IBILCE/UNESP, 15054-000. São José do Rio Preto, SP, Brazil.
ABSTRACT: The flutamide antiandrogenic effects on the Guinea pig male prostate morphology in puberal, post-puberal and adult ages were evaluated in the present study. Daily-treated group animals received flutamide subcutaneous injection at a dose of 10 mg/Kg body weight for 10 days. The control group animals received a pharmacological vehicle under the same conditions. The lateral prostate was removed, fixed and processed for light and transmission electron microscopy. The results revealed an increase of the acinus diameter in the treated puberal animals and straitness in the stromal compartment around the acini. The epithelial cells exhibited cubic phenotype. In the post-puberal and adult animals, a decrease of the acinus diameter was observed, as well as an increase of the smooth muscle layer and presence of the folds at epithelium. The ultrastructural evaluation of the secretory cells in the treated group demonstrated endomembrane enlargement, mainly in the rough endoplasmic reticulum and Golgi apparatus. In addition, a decrease of the microvilli and alterations in the distribution patterns and density of the stromal fibrillar components were observed. In conclusion, the flutamide treatment exerts tissue effects on the lateral prostate, promoting stroma/epithelium alterations.
Keywords: Lateral Prostate. Flutamide. Antiandrogen. Secretory Cells.
Address correspondence to: Dr. Sebastião Roberto Taboga. Departamento de Biologia, IBILCE/UNESP, Rua Cristóvão Colombo, 2265, Jardim Nazareth, São José do Rio Preto, SP, BRAZIL. Zipcode: 15054-000. Tel: (+55 17) 2212386; Fax: (+55 17) 2212390. E-mail: taboga@bio.ibilce.unesp.br
Introduction
The differentiation and maintenance of the prostate functional integrity and other male reproductive system accessory organs are dependent on circulating androgen levels. Reciprocal interactions between the mesenquima/epithelium and stroma/epithelium take place in the embryo and in the adult animals, respectively (Price, 1963; Aumüller and Seitz, 1990; Rosai, 1996; Hayward et al., 1997; Thomson et al., 1997).
During the aging processes, most histological alterations occur in the prostate gland, in response to the hormonal variation during this life period (Rosai, 1996). In men, the main alterations are related to severe diseases, leading to the urinary retention, the well-known benign prostatic hyperplasia (Droller, 1997). In addition, old men can develop prostate malignant lesions, such as adenocarcinomas, existing the genetic predisposition by these lesions (Hayward et al., 1997). Such lesions, malignant or not, can be treated by the androgen removal strategies (Price, 1963; Colombel and Buttyan, 1995; Droller, 1997; Rauch et al., 1997).
Flutamide has been a potent nonsteroidal antiandrogen unctionally specific for androgen-dependent accessory sex glands, mainly in the treatment of prostatic carcinoma (Sogani et al., 1984; Kassim et al., 1997; Lucas et al., 1997). This drug blocks the action of the biologically active testosterone metabolite, dihydrotestosterone, on the prostatic tissue androgen receptors and inhibit the testosterone-stimulated prostatic DNA synthesis (Gordon et al., 1984; Marchetti and Labrie, 1988).
The events associated with modifications in the prostate epithelium and stroma were investigated after the orchiectomy (Kofoed et al., 1990; Carvalho and Line, 1996; Carvalho et al., 1997a,b; Vilamaior et al., 2000). The morphologic and ultrastructural modifications in the epithelium and stroma still need more investigations after antiandrogen therapy, mainly as an attempt to understanding the prostate epithelium-stroma relationships. Thus, the aim of this work was to evaluate the morphological and ultrastructural aspects of the flutamide effects, on the prostate epithelial and stromal regions in
Material and Methods
Experimental Design
Thirty Guinea pigs Cavia porcellus were divided, randomly, into 3 groups of 10 animals each, where each group corresponded to the phase of the postnatal development: group 1 - puberal phase (between 15 and 60 days after birth), group 2 - post-puberal phase (between 60 and 120 days after birth) and group 3 - adult phase (between 120 and 360 days after birth). In each group, 5 animals (experimental groups) received randomly for 10 days, subcutaneous injections of 0.5 ml of flutamide 10 mg/Kg/body weight (Signa Chemical Co,
Twenty-four hours after the last injection, the animals in both control and experimental groups, were sacrificed by ether anesthesia and lateral prostate fragments were removed and processed for the light and electron transmission microscopy techniques.
Light Microscopy
Lateral prostatic fragments were fixed by immersion in Karnoviskys fixative, (5% paraformaldehyde and 2.5% glutaraldehyde solution in Sörensën phosphate buffer at pH 7.3) during 24 h. After this procedure, tissues were dehydrated in crescent series of ethanol and embedded in glycol methacrylate resin (Leica historesin embedding kit) and sectioned at 3 mm on a Leica microtome.
The histological sections were stained by the haematoxylin-eosin (H&E) (Behmer et al., 1976) for general analysis and 0.025% Toluidine Blue solution in Mc Ilvaine buffer at 4.0 pH histochemical method was used to characterize the nuclear phenotypes in the prostate epithelium (Mello and Vidal, 1980). The morphological and morphometric-stereological analyses were employed in the distal portion of the prostatic ductal system. The preparations were observed with either a Zeiss Jenaval or an
Transmission Electron Microscopy
Lateral prostate fragments were fixed in 3% glutaraldehyde plus 0.25% tannic acid in Millonigs buffer at 7.3 pH, containing 0.5% glucose during 24 hours (Cotta-Pereira et al., 1976). After washing with the same buffer, the material was post-fixed in 1% osmium tetroxide (1 h), washed again, dehydrated in graded acetone and embedded in araldite resin (Cotta-Pereira et al., 1976). Ultrathin silver sections were obtained using a diamond knife on an LKB ultramicrotome and contrasted with 2% alcoholic uranil acetate (Watson, 1958) and 2% lead citrate in sodium hydroxide solution for 10 min (Venable and Coggeshall, 1965). Grids were examined under a Zeiss-Leo 906 transmission electron microscope operating at 80 kV of the
Morphometric-stereological analysis
Using a system of images analysis (Image Pro-Plus, Media Cybernetics), H&E sections were analyzed. Images of 30 histological fields for each experimental group in the ages studied were analyzed, so that histological fragments of all animals were equally evaluated. The morphometric-stereological analyses were obtained by Weibels mutipurpose graticulate with 120 points and 60 test line (Weibel, 1978) to compare the relative proportion of the prostatic components in the different ages in both experimental groups.
The proportions obtained as percentages were submitted to the ANOVA test, where P < 0.05 indicated a significant difference.
Results
Control Group
The histological analysis revealed that the Guinea pig lateral prostate is a tubulo-acinar gland. This gland is constituted of acini surrounded by connective tissue (nonmuscle stroma) in the epithelial basis, and smooth muscle cells (muscle stroma), which associate themselves to form an arrangement of concentric layers involving the epithelial portions in all its extension (Figs. 1, 3 and 5). The interacinar space is constituted by a stroma of loose connective tissue (Figs. 1, 5).

FIGURES 1 to 12.
Prostatic histological sections of male guinea pig embedded in historesin and stained by H&E (Figs. 1 to 6) and by Toluidine Blue (Figs. 7 to 12). Figs.: 1, 3, 5, 7, 9 and 11 - Prostate of the control animals. Prostatic acinus of the puberal animals (Figs. 1 and 7) constituted by epithelium simple columnar cells (ep), with basal nuclei and surrounded by a concentric and thickens of smooth muscle fibers layer (m) and for a nonmuscle stroma (*) in the epithelium base. Lumen (L). Figs. 1 = X 290 and 7 = X 580. - Postpuberal phase (Figs. 3 and 9) with acini constituted by columnar or cuboidal epithelium with small folds and basal nuclei and lined by smooth muscle fibers layer. Note the clear area in the supranuclear area probably occupied by the Golgi complex (arrowhead). Figs. 3 = X 290 and 9 = X 580. The acini of adult animals (Figs. 5 and 11) are wide and constituted by simple epithelium of low cells without any form folding and delineated by a strait smooth muscle layer. Among the secretory acini there is a stroma constituted by a connective loose tissue (ct). Figs. 5 = X 232 and 9 = X 580. Figs. 2, 4, 6, 8, 10 and 12 - Prostate of the treated animals. In the puberal (Figs. 2 and 8) and postpuberal animals (Figs. 4 and 10), note an apparent decrease in the thickness of smooth muscle layer and little folds in the epithelium, resulting in an increase of the acini diameter and a decrease of the height epithelial cells presenting basal nuclei. The probable area of the Golgi is reduced (arrowhead). Figs. 2 and 4 = X 290, 8 and 10 = X 580. The acini from the adult animals (Figs. 6 and 12) has an apparent decrease of its diameter and show a columnar simple epithelium forming some folds, with an increase of height of the epithelial cells and of the thickness of the smooth muscle. Figs. 6 = X 232, and 12 = X 580.
The stromal compartment, adjacent to the epithelium, is constituted by fine collagen and elastin fibers (Figs. 22, 23, 24 and 30). In this matrix, fibroblasts and smooth muscle cells are inserted (Figs. 28 and 29).
The epithelial cells exhibited the rough endoplasmic reticulum and Golgi apparatus with well-developed secretory granules and occupying the cytoplasmic apical region, which through light microscopy by H&E, it is a cromophobous region. The nuclei were oval to oblong in shape and were located in the central region of the cells (Figs. 9, 11, 13, 15, 16 and 17). The epithelial cells, intercepted at the base by the smaller, basal cells, rested on a thin, slightly undulating basal lamina and separated it from the connective tissue and smooth muscle cells (Figs. 22, 23 and 24). The prominent feature of the luminal membrane was the presence of numerous packed microvilli (Figs. 13 and 14).
Flutamide-treated group
The flutamide therapy provoked some alterations on the Guinea pig lateral prostate region. The epithelial cells seem to respond differently to the flutamide and in same acini responsive cells could be found beside refractory cells to the antiandrogen (Fig. 18).
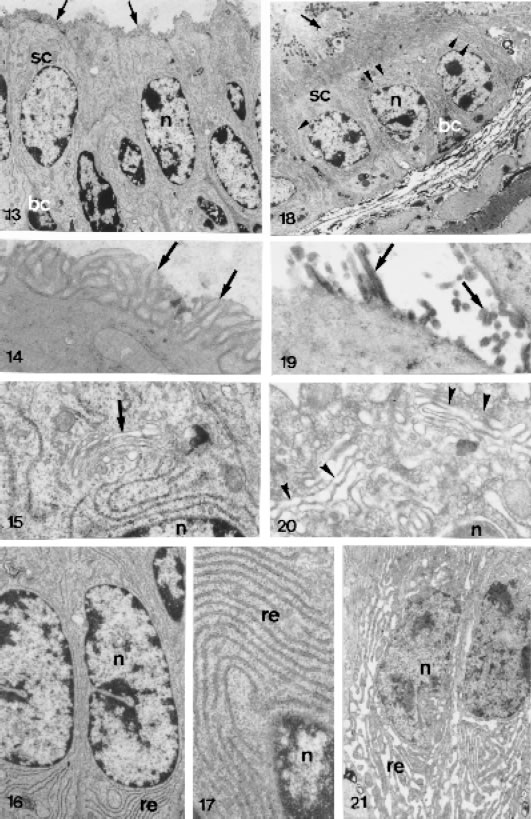
FIGURES 13 to 21.
Ultrastructural section male lateral prostatic of control (Figs. 13 to 17) and treated (Figs. 18 to 21) animals. Fig. 13 - Portion of glandular epithelium showing the secretory acinar cells (sc) with numerous microvilli (arrow) X 4,676. Fig. 18 - Secretory epithelium with enlarged endomembranes (arrowhead). Basal cell (bc). Nucleus of the epithelial cell (n). Secretory granules (g) X 3,018. Figs. 14 and 19 - Apical cytoplasm showing abundant arrangement of the microvilli (arrows) in the control animals and its shortage in the treated animals. Figs. 14 = X 24,025 and 19 = X 34,800. Fig. 15 - Golgi complex (arrow) above the nucleus (n) in the epithelial cell of the control animals X 22,301. Fig. 20 - Golgi complex with dilated cisternae after the treatment X 32,325. Figs. 16, 17 and 21 - Cytoplasm of the epithelial cells showing the membranes of the rough endoplasmic reticulum (re) in the control animals (Figs. 16 and 17) and its dilation after the treatment (Fig. 21). Figs. 16 = X 11,400, 17 = X 21,710 and 21 = X 83,52.
The responsive epithelial cells demonstrated an endomembrane enlargement, mainly in the rough endoplasmic reticulum and Golgi apparatus. Furthermore, a decrease of the microvilli was observed in all treated groups (Figs. 18, 19, 20 and 21). The prominent feature of the apical cytoplasm was the presence of numerous granular secretory granules of different sizes, probably some representing the condensing vacuoles of the Golgi complex. Occasionally these secretory granules liberate its content in the acinus lumen (Fig. 18).
The androgen blockade also provoked alterations in the distribution patterns and density of the fibrillar component that compose the connective tissue where the prostatic acini rest. The results showed an irregular distribution and an apparent decrease in the concentration of the collagen fibers, mainly in the base of the epithelium (Figs. 25, 26 and 27) and between the smooth muscle cells that surround the acini (Figs. 31, 32 and 33). The elastic system fibers are thinner and have irregular distribution and concentration (Figs. 34).

FIGURES 22 to 27.
Ultrastructural section male lateral prostatic of control (Figs. 22, 23 and 24) and treated (Figs. 25, 26 and 27) animals, showing the nonmuscle stroma (nm) in the epithelial base. Figs. 22, 23 and 24 - Note great amount of collagen fibers (c) along every of the epithelium base. Figs. 25, 26 and 27 - Prostate of the treated animals. A considerable decrease of collagen fibers was observed in the nonmuscle stroma. Glandular epithelium (ep). Basal lamina (arrowheads). Smooth muscle fiber (m). Fibroblast (f). Figs. 22 = X 17,400, 23 = X 38,000, 24 = X 17,000, 25 = X 13,800, 26 = X 17,654, 27 = X 13,308.
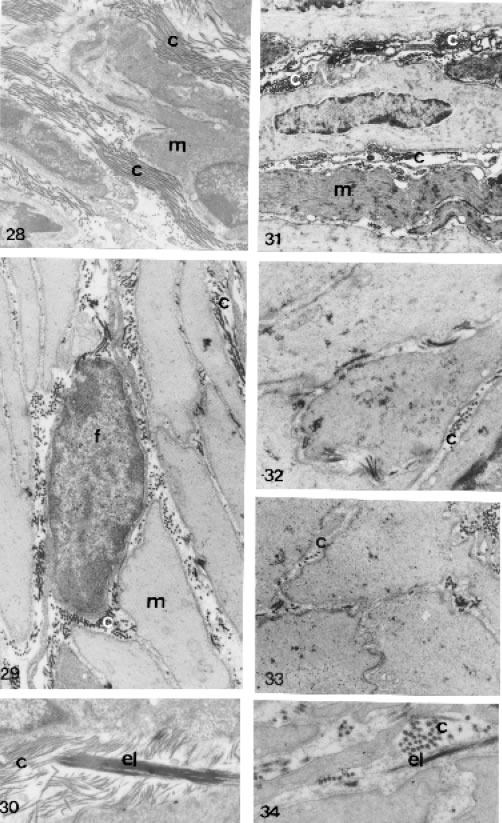
FIGURES 28 to 34.
Ultrastructural section male lateral prostatic of control (Figs. 28, 29 and 30) and treated (Figs. 31, 32, 33 and 34) animals, showing in the interacini stromal and association of the smooth muscle, collagens and elastic fibers. Figs. 28, 29 and 30 - Prostate of control animals with great amounts of collagen fibers (c) and thick elastic system fibers (el), both intermingling in smooth muscle fibers (m). Figs. 28 = X 7800; 29 = X 12,000 and 30 = X 20,926. Figs. 31, 32, 33 and 34 - Prostatic interacini stroma of treated animals showing a drastic decrease of the collagen fibers and elastic system fibers, which become fine (el). Fibroblast (f). Figs. 31 = X 7913, 32 = X 18,000; 33 = X 15,000 and 34 = X 25,050.
The morphologic and morphometric analyses in lateral prostate of the puberal animals demonstrated a significant increase of the acini diameter, promoting a straitness of the connective tissue and smooth muscle cells layer (Table 1). The epithelial cells exhibited cubic phenotype and the nucleus to occupies almost all area of the cytoplasmic space (Figs. 2 and 8). In these cells the nucleoli are more conspicuous than in control group animals.
In the post pubertal and adult animals, decreases of the acinus diameter and folds in the acinar epithelium were observed (Table 1). In addition, an increase in the thickness of smooth muscle layer was noticed in these ages. Apparently, there was a reduction in the height of the epithelial cells, presenting nuclei occupying almost the whole cell and apparent decrease of the area occupied by the Golgi complex in the post pubertal animals (Figs. 4 and 10).

Discussion
In this study the administration of 10mg/Kg of flutamide was enough to promote significant morphologic and ultrastructural alterations in distribution pattern and in concentration of the fibrillar components (collagen, reticular and elastic system fibers) and cellular components of Guinea pig lateral prostate according to the age phase of the postnatal development.
The epithelial cells showed different phenotypes in each phase of the development after the flutamide treatment. In the puberal phase, where the amount of androgens acting on the prostate is smaller (Zhao et al., 1993; Horsfall et al., 1994), the glandular epithelium exhibits cubic cells with nucleus occupying almost the whole cell and area luminal extensive. In this case, despite the low levels of androgens, the amount of administered flutamide was enough to promote the considerable morphologic and structure alterations in the prostatic gland architecture.
In the adult phase, the androgens maintain the morphology and the function in the prostatic tissue, with a rate of extremely low proliferation, which is balanced with the cellular death (Cunha et al., 1996).
There is a certain variation in the hormonal levels with the advancement of the age, mainly of testosterone levels produced by the testicles. In this case, the flutamide therapy was capable of blocking the androgen levels that are acting in this organ by competition with androgen receptors.
In the post-puberal phase, where the levels of circulating androgens are higher (Zhao et al., 1993; Horsfall et al., 1994), there was a major competition with the flutamide for the androgens receptor presenting, therefore, an moderated effect in the prostatic tissue compartments when compared with the other ages. Acini were constituted by epithelial cubic and columnar cells with small folds, lumen with little dilation and an increase of the smooth muscle layer, which was not significant.
Besides, the androgen blockade provoked a decrease in the concentration and thickness, as well as a rearrangement of the collagen and elastic fibers among the muscle fibers mainly in the epithelial base. These modifications are related to tissue contraction and structure maintenance of other components of extracellular matrix during the remodeling/involution process under the effect of the flutamide.
Our data demonstrated that the proportion of stroma is major during puberty; with the advancement of the age, an apparent increase of the acini diameter and tubules in this species, was observed. In intact animals an increase of the acini diameter, promoting a straitness of the connective tissue and smooth muscle cells layer, was observed.
Zhao et al. (1993), using a prostate gland microdissection technique described by Sugimura et al. (1986a), carried out a preliminary study of ductal morphogenesis of the guinea pig prostatic complex. This technique revealed that branching morphogenesis of this species is virtually completed at birth, with no significant increase in number of branches and ductal tips thereafter. The postnatal growth is accomplished mainly by ductal network elongation (puberal phase) with a little additional branching (post-puberal phase) but with an increase in size (volume) of the tubules (adult phase).
These small modifications in the structures of Guinea pig ductal network after the postnatal development are directly linked to the dependence of this organ to the levels of circulating androgens and acts in each phase of the development. Due to this variation in the androgen concentration in each phase, the detailed morphologic description in alterations of the three different types of acini found in the prostate of Guinea pig males along the postnatal development, after blocked hormonal, could be made. Thus, in the ages where the androgens concentration is small (puberal phase) and where its concentration is fundamental to maintain the prostatic functional and secretory integrity in normal levels (adult phase), the flutamide exhibited its efficient antiandrogenic activity by inhibiting the mechanisms of prostatic androgen receptor activation and function, promoting alterations in the epithelial acinar cells and in the stroma elements. An opposite process was observed in the post-puberal animal prostate, where the androgen concentration is major, and therefore there was a hormonal blockade smaller in the tissues by the flutamide.
The differentiated action of the flutamide in the several phases of the postnatal development seems to be directly linked to the hormonal influence which is either absolute (in the adult phase and post-puberal) or relative or almost nonexistent (in the puberal phase), (Dounjacour and Cunha, 1988).
Due to the block of testosterone action and its metabolite dihydrotestosterone, on the prostatic tissue by flutamide and also by the heterogeneous distribution of the androgen receptors in the epithelium and stroma (Galibraith and Duchesne, 1997), the secretory cells responded differently to treatment, presenting normal ultrastructural characteristics, interposed with cells which underwent the hormonal blockade of the flutamide.
The structural and ultra-structural alterations in Guinea pig prostatic architecture after the blockade androgen by the flutamide have been explained based in three action mechanisms of the nonsteroidal antiandrogen drugs (Burton and Trachtenberg, 1986): (1) Decrease binding of dihydrotestosterone to the receptor by competitive inhibition. (2) Decrease of androgen receptors concentration. (3) Decrease of 5a-redutase enzyme activity in the conversion of the testosterone to dihydrotestosterone.
By the ultrastructural alterations observed in this study, an increase of LH and consequently of the testosterone levels probably occurred, interfering in the flutamide action upon prostatic androgen receptors.
In addition, the stereologic-morphometrical analysis of the Guinea pig lateral prostate alterations caused by flutamide allow us to assume that flutamide has an effective action in the inhibition of the normal prostate growth. This drug promotes also significant alterations in the cell differentiation pattern and maintenance of the morphofunctional integrity of the adult gland.
Acknowledgements
The authors are indebted to the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP for RSC and WRS fellowships and Grants to SRT - Process n° 00/06146-7. Special acknowledgments are attributed to Mr. Luiz Roberto Falleiros Junior and Ms. Rosana Silistino de Souza, MSc for the technical assistance. Special acknowledgements to Dr. A.L. Hattnher for English review, to Dr. Maria Astride Saad Corradi and Lara Silvia Corradi for the suggestions and advice.
References
Aumüller G, Seitz J (1990). Protein secretion and secretory process in male accessory sex glands. Int Rev Cytol, 121: 127-231. [ Links ]
Behmer OA, Tolosa EMC, Freitas-Neto AG (1976). Manual para histologia normal e patológica. Edart-Edusp, São Paulo, 225p. [ Links ]
Burton S, Trachtenberg J (1986). Effectiveness of antiandrogens in the Rat. J Urol, 136: 932-935. [ Links ]
Carvalho HF, Line SRP (1996). Basement membrane associated changes in the rat ventral prostate following castration. Cell Biol Int, 20: 809-819. [ Links ]
Carvalho HF, Vilamaior PSL, Taboga SR (1997a). Elastic system fibers of the rat ventral prostate and its modifications following orchiectomy. Prostate 32: 27-34. [ Links ]
Carvalho HF, Vilamaior PSL, Taboga SR (1997b). Collagen type VI is a component of the extracellular matrix microfibrils network of the prostatic stroma. Tissue Cell 29: 163-170. [ Links ]
Colombel MC, Buttyan R (1995). Hormonal control of apoptosis: the rat prostate gland as a model system. In: Schwartz LM, Osborne, BA, eds. Methods in Cell Biology - Cell Death. V. 46. Academic Press, New York, p. 369-385. [ Links ]
Cotta-Pereira G, Rodrigo FG, David-Ferreira JF (1976). The use of tannic acid-glutaraldehyde in the study of elastic related fibers. Stain Technol 51: 7-11. [ Links ]
Cunha GR, Hayward SW, Dahiya R, Foster BA (1996). Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat 155: 63-72. [ Links ]
Dounjacour AA, Cunha GR (1988). The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol 128: 1-14. [ Links ]
Droller MJ (1997). Medical approaches in the management of prostatic disease. Br J Urol, 79 (Suppl.) 2: 42-52. [ Links ]
Galibraith SM, Duchesne GM (1997). Androgens and prostate cancer: Biology, pathology and hormonal therapy. Eur J Cancer 33(4): 545-554. [ Links ]
Gordon AS, Susan JA, Richard GM (1984). A Pilot study of flutamide. Med J Aust 140: 219-221. [ Links ]
Hayward SW, Rosen MA, Cunha GR (1997). Stromal-epithelial interactions in the normal and neoplasic prostate. Br J Urol 79 (Suppl.2): 18-26. [ Links ]
Horsfall DJ, Mayne K, Ricciardelli C, Rao M, Skinner JM, Henderson DW, Marshall VR, Tilley WD (1994). Age-related in guinea pig prostatic stroma. Lab Invest 70: 753- 763. [ Links ]
Kassim NM, McDoland SW, Reid O, Bennett NK, Gilmore DP, Payne AP (1997). The effects of pre- and postnatal exposure to the nonsteroidal antiandrogen flutamide on testis descent and morphology in the Albino Swiss rat. J Anat 190(4): 577-588. [ Links ]
Kofoed JÁ, Tumilasci OR, Curbelo HM, Fernandes Lemos SM, Arias NH, Houssay AB (1990). Effect of castration and androgens upon prostatic proteoglycans in rats. Prostate 16: 93-102. [ Links ]
Lucas JC, Renfree MB, Shaw G, Butler CM, (1997). The influence of anti-androgen flutamide on early sexual differentiation of the marsupial male. J Reprod Fertil 109: 205-212. [ Links ]
Marchetti B, Labrie F (1988). Characteristics of flutamide action on prostatic and testicular functions in rat. J Steroid Biochem 29(6): 691-698. [ Links ]
Mello MLS, Vidal BC (1980). Práticas de biologia celular. Edgard Blücher-Funcamp, Campinas, 71p. [ Links ]
Price D (1963). Comparative aspects of development and structure in the prostate. Natl Cancer Inst Monogr 12: 1-27. [ Links ]
Rauch F, Polzar B, Stephan H, Zanotti S, Paddenberg R, Mannherz HG (1997). Androgen ablation leads to an upregulation and intranuclear accumulation of deoxyribonuclease I in rat prostate epithelial cells paralleling their apoptotic elimination. J Cell Biol, 137: 909-923. [ Links ]
Rosai J (1996). Male reproductive system. In: Ackermans Surgical Pathology. 8ed. ROSAI J, eds. Mosby-Year, St. Louis-USA, Vol. I, p. 1221-1256. [ Links ]
Sogani PC, Vagaiwala MR, Whitmore Jr WF (1984). Experience with flutamide in patients with advanced prostatic cancer without prior endocrine therapy. Cancer 54: 744-550. [ Links ]
Sugimura Y, Cunha GR, Donjacour AA (1986a). Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 34: 961-971. [ Links ]
Thomson AA, Foster BA, Cunha GR (1997). Analyses of growth factor and receptor mRNA levels during development of the rat seminal vesicle and prostate. Development 124: 2431-2439. [ Links ]
Venable JH, Coggeshall R (1965). A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407-408. [ Links ]
Vilamaior PSL, Felisbino SL, Taboga SR (2000). Collagen fiber reorganization in the rat ventral prostate following androgen deprivation. A possible role for smooth muscle cell. Prostate 45(3): 253-258. [ Links ]
Watson ML (1958). Staining tissue sections of electron microscopy with heavy metals. J Biophys Biochem Cytol 4: 475-478. [ Links ]
Weibel ER (1978). Principles and methods for the morphometric study of the lung and other organs. Lab Invest, 12: 131-155. [ Links ]
Zhao CY, Tam CC, Wong YC (1993). Morphogenesis and ductal development of the prostatic complex of the guinea pig. J Morphol, 217: 219-227. [ Links ]
Received on February 5, 2003.
Accepted on Dicember 2, 2003.














