Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.28 n.1 Mendoza ene./abr. 2004
Bacteriostatic action of synthetic polyhydroxylated chalcones against Escherichia coli
María De Los Angeles Alvarez, Valeria E. P. Zarelli, Nora B.Pappano And Nora B. Debattista
Laboratory of Physical-Chemistry, Department of Chemistry, National University of San Luis. Chacabuco 917. (5700) San Luis, Argentina.
Abstract: In previous work the bacteriostatic action of trihydroxylated chalcones against Staphylococcus aureus ATCC 25 923 was investigated. In this work the action of 2´,4´,2-(OH)3-chalcone, 2´,4´,3-(OH)3- chalcone and 2´,4´,4-(OH)3 –chalcone against Escherichia coli ATCC 25 922 was evaluated. Growth kinetic curves of E.coli were made in nutritive broth added with increasing drug concentrations. The specific growth rates of the microorganisms were calculated by a kinetic turbidimetric method, which was previously probed and the minimal inhibitory concentrations (MIC´s) were evaluated by a mechanism of action proposed. The MICs of 2´,4´,3-(OH)3-chalcone and 2´,4´,2-(OH)3-chalcone were 46 mg/ml and 122 mg/ml, respectively. The 2´,4´,4-(OH)3–chalcone was inactive. The MIC value of 2´, 4´, 3-(OH) 3-chalcone (46 mg/ml), more active than 2´, 3-(OH) 2-chalcone (72.2 mg/ml) may be due to the introduction of an electron donating group (-OH) at position 4´ in the aromatic A- ring, which activates the region that includes the 2´-hydroxyl neighbur group and the a,b- unsaturated carbonyl group.
Key words: Chalcones. Bacteriostatic Activity. Hydroxylation.
Address correspondence to: Dra. Nora B. Pappano. Laboratory of Physical-Chemistry, Department of Chemistry, San Luis National University. Chacabuco 917, (5700) San Luis, ARGENTINA. Phone: (+54-2652) 424689 int. 122; Fax: (+54- 3652) 422644; E-mail: npappano@unsl.edu.ar
Introduction
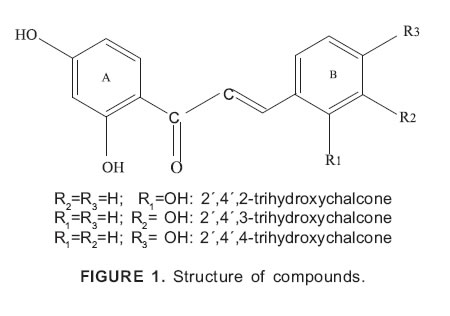
A great number of natural flavonoids with various biological activities have been identified in the last years (Czinner et al., 1999; Hernández and Prieto, 1999; Wächter et al., 1999). In this family, several compounds show biological activities such as: antimicrobial (Gafner et al., 1996; Li et al., 1998; Ravha et al., 2000; Olivella et al., 2001), antiviral (Kurokawa et al., 1995; Sindabiwe et al., 1999), anti-inflammatory (Wiseman and Hallywell, 1996; Middleton et al., 2000) and other therapeutic applications (So et al., 1996; Zi and Agarwal, 1999; Evans, 2000). The increase of bacteriostatic action due to the presence of free hydroxyl groups into the chalcone molecule has been previously demonstrated (Pappano et al., 1990, 1994). It was found that the presence of hydroxyl groups, especially at 4 and 4´ positions of 2 ´- hydroxychalcone enlarges the bacteriostatic activity against Staphylococcus aureus ATCC 25 923 (Pappano et al., 1990; Devia et al., 1998). In the present work we investigate whether trihydroxylated chalcones possess greater bacteriostatic action against Escherichia coli ATCC 25 922 than the dihydroxylated ones previously tested (Pappano et al., 1994). Minimal inhibitory concentrations (MICs) of 2´,4´,2-trihydroxychalcone (I), 2´,4´,3-trihydroxychalcone (II) and 2´,4´,4-trihydroxychalcone (III) against the microorganism were evaluated using a kinetic turbidimetric method (Pappano et al., 1990).
Materials and Methods
Microbial strain:
E. coli ATCC 25 922 (acquired in American Type Culture Collection) maintained by successive subcultures in trypticase soy agar (BBL) at 4ºC and by liofilization.
Chemicals:
High purity compounds were employed: 2´,4´,2- trihydroxychalcone (I) 2´,4´,3-trihydroxychalcone (II)and 2´,4´,4-trihydroxychalcone (III) were prepared by Claisen-Schmidt condensation (Dhar, 1981). Their structures (Fig. 1) were determined by the chromatographic and spectroscopic data: Rf (TLC): 0.174; UVlmax (MeOH) nm: 368; 242; 205; 1H NMR and 13C NMR (Devia et al., 1998).
Turbidimetric kinetic method:
A 24 h culture of E.coli ATCC 25 922 in slant agar were transferred to 30 ml of Müller- Hinton broth (Oxoid) and incubated for 18 h at 35ºC with permanent stirring, in order to be used as inoculum. Erlenmeyers containing 100 ml of culture medium with increasing drug concentrations to be tested were inoculated with 2 ml of inoculum and stirred in a Rosi 1000 culture chamber at 35ºC and 180 rpm, including one without drug as control. Aliquots were extracted at 20 min intervals during 5 h and transmittance (T) was registered in a UVVisible recording spectrophotometer Shimadzu 160 A. T values were related to the number cfu/ml (colony forming units/ml) Nt, through the expression for E. coli (Pappano et al., 1990):
ln Nt = 27.1 – 8.56 . T (1)
Results
Compounds assayed were efficient against E. coli ATCC 25 922. The number of cfu/ml at different timeswas obtained by the expression of the turbidimetric kinetic method.
Considering the microbial growth law
ln Nt = ln No + m . t (2)
where t: time in min; No: cfu/ml at t = 0; Nt : cfu/ml at time t; m: specific growth rate in min–1, values for E. coli specific growth rates in media with increasing drug concentrations were obtained from the ln Nt vs. t plot in the exponential growth phase. Results from the E. coli growth tests in presence of 2´,4´,3 - trihydroxychalcone are shown in Figure 2.
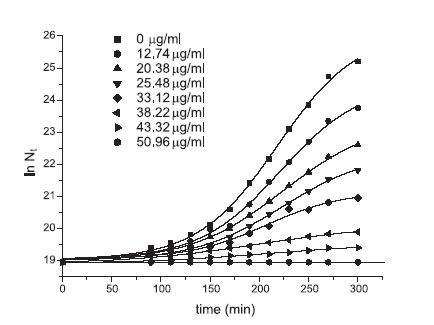
FIGURE 2. rowth of Escherichia coli ATCC 25 922 in media containing 2´,4´,3- trihydroxychalcone, at the indicated concentration.
In Table 1 values of T, ln Nt (evaluated from equation 1) and t are informed.

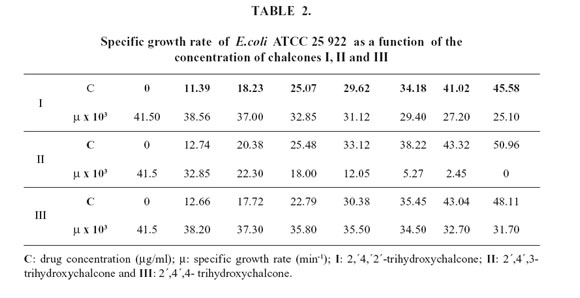
Table 2 exhibits values for the microbial specific growth rates and the drug concentrations added to the culture media.
Discussion
The results were interpreted satisfactorily by means of the bacteriostatic inhibition mechanism previously proposed for dihydroxylated chalcones (Pappano et al., 1994). Thus, the variation of the specific growth rate (m) with the drug concentration follows the relation
m = m T - k . C (3)
where m: specific growth rate (min-1); mT: specific growth rate in medium without drug (min-1) (control); k: specific inhibition rate (ml /(mg.min)) and C: drug concentration (mg/ml).
The graphical representation of equation (3) is shown in Figure 3 for the assayed compound and minimal inhibitory concentrations were evaluated by extrapolation at m = 0.

FIGURE 3. Graphical determination of MICs (minimal inhibitory concentrations) by extrapolation at the abcis when m = 0
The minimal inhibitory concentration (MIC) values obtained for the compounds tested in our study were as follows: 2´,4´,3-trihydroxychalcone 46 mg/ml <<< 2´,4´,2-trihydroxychalcone 122 mg/ml <<< 2´,4´,4-trihydroxychalcone inactive.
These values allowed us to conclude that the presence of a new OH group at 4´ position in the structure of 2´, 3-dihydroxychalcone (MIC = 72.2 mg/ml) increases the bacteriostatic activity of the base compound. The increase of efficacy on the part of 2´,4´,3- trihydroxychalcone could be attributed to the presence of a OH group at 4´ position in connection with the active region that comprises the –OH at 2´ position and the a,b unsaturated carbonyl group. On the other side, it was found that the presence of a OH group in 4 position of 2´,4´,4-trihydroxychalcone, in contrast to the results of the same chalcones against Staphylococcus aureus (Devia et al., 1998), annuls the action of the same one. The similar behavior was observed for 2´,4´,2- trihydroxychalcone regarding 2´,4´-dihydroxychalcone. Different wall structure of Gram positive and Gram negative bacteria could be the reason of those results.
Acknowledgements
The present work was supported by the National University of San Luis.
References
Czinner E, Kéry Á, Hagymás K, Blázovics A, Lugasi A, Szõke É, Lemberkovics É (1999). Biologically active compounds of Helychrysum arenarium (L.) Moench. Eur J Drug Metab Pharmacokinet, 24: 309-313. [ Links ]
Devia CM, Pappano NB, Debattista NB (1998). Structure-Biological activity relationship of synthetic trihydroxylated chalcones. Rev Microbiol, 29: 307-310. [ Links ]
Dhar DN (1981). The Chemistry of Chalcones and Related Compounds. John Wiley & Sons, New York, pp. 5-9. [ Links ]
Evans AN (2000). Influence of dietary components on the gastrointestinal metabolism and transport of drugs. Therapeutic Drug Monitoring, 22: 131-136. [ Links ]
Gafner S, Wolfender JL, Mavi S, Hostettmann K (1996). Antifungal and antibacterial chalcones from Myrica Serrata. Planta Med, 62: 67-69. [ Links ]
Hernández A, Prieto E (1999). Plantas que contienen polifenoles. Antioxidantes dentro del estilo de vida. Rev Cubana Invest Biomed, 18: 12-14. [ Links ]
Kurokawa M, Nagasaha K, Hirabayashi T (1995). Efficacy of traditional herbal Medicines in combination with Acyclovir against Herpes Simplex virus Type 1 infection in vitro and in vivo. Antiviral Res, 27: 19-37. [ Links ]
Li W, Asada Y, Yoshikawa T (1998). Antimicrobial flavonoids from Glycyrrhiza glabrharry root cultures. Planta Med, 64: 746-747. [ Links ]
Middleton EJN, Kandaswmi C, Theoharides TC (2000). The effects of plant flavonoids on mammalian cells: implications for inflammation, hearth disease and cancer. Pharmacol Rev, 52: 673-751. [ Links ]
Olivella M, Zarelli VEP, Pappano NB, Debattista NB (2001). A comparative study of bacteriostatic activity of synthetic hydroxylated flavonoids. Brazilian J Microbiol, 32: 229-232. [ Links ]
Pappano NB, Centorbi OP, Ferretti FH (1990). Determinación de la concentración inhibitoria mínima a partir de parámetros cinéticos de crecimiento. Rev Microbiol, 21: 183-188. [ Links ]
Pappano NB, Centorbi OP, Ferretti FH (1994). Determination of the responsible molecular zone for the chalcones bacteriostatic activity. Rev Microbiol, 25: 168-174. [ Links ]
Ravha JP, Remes S, Heinonen M, Hopia A, Kähkömen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P (2000). Antimicrobial effects of Finnish plant extracts Containing flavonoids and other phenolic compounds. Int J Food Microbiol, 56: 3-12. [ Links ]
Sindabiwe JB, Calomme M, Cos P, Totté J, Pieters L, Vlietinck A, Vanden Berghe D (1999). Screening of seven selected Roandan medicinal plants for antimicrobial and antiviral activities. J Etnopharmacol, 65: 71-77. [ Links ]
So FV, Guthier N, Chambers AS, Mousa M, Carroll KK (1996). Inhibition of human breast cancer cell proliferation and delay mammary tumorigenesis by flavonoids and Citrus juices. Nutrition and Cancer 26: 167-181. [ Links ]
Wiseman H, Hallywell B (1996). Damage to DNA by reactive oxygen and nitrogen Species: role in inflammatory disease and progression to cancer. Biochem J, 313: 17-29. [ Links ]
Wächter GA, Wangmaneerat A, Caple KM, Montenegro G, Timmermann BN (1999). Flavonoids and terpenoids from Luma gayana (Barn.) Burret. Z Naturforsch, 54C: 1140-1142. [ Links ]
Zi XL, Agarwal R (1999). Modulation of mitogen-activated protein kinase activation and cell cycle regulators by the potent skin cancer preventive agent silymarin. Biochem Biophys Res Comm, 263: 528-53. [ Links ]
Received on May 16, 2003.
Accepted on September 19, 2003.














