Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.28 n.2 Mendoza abr./ago. 2004
Nilza Cristina Buttow, Miriam Santin, Luciana Conci Macedo, Aline Cristina Neres Teixeira, Gisele Caroline Novakowski, Taíse Roberta Bolonheis Armelin and Kathya Assmann
Department of Morphophysiological Sciences; Universidade Estadual de Maringá; Brazil
Address correspondence to: Dra. Nilza Cristina Buttow. Departamento de Ciências Morfofisiológicas, Universidad Estadual de Maringá. Av. Colombo, 5790 Bloco H-79 – CEP 87020-900. Maringá, PR, BRASIL. FAX 00 55 44 261 4340. E-mail: ncbuttow@uem.br
Abstract: A morphological and quantitative study in the ileal and colonic myenteric and submucous plexuses of rats after BAC denervation was performed. Four groups were employed: SI – ileum control; CBI – denervated ileum; SC – colon control; and CBC – denervated colon. We used the Myosin-V immunohistochemistry technique to study the myenteric and submucous plexuses. In the submucous plexus of the ileum and colon there was not a significant decrease in the number of neurons/mm2 and of ganglia/mm2. The denervation of the myenteric plexus in the group CBI was 44.7% and in the group CBC, 68.3%. In the myenteric plexus there was also a significant decrease in the number of ganglia/mm2 (13.8% in group CBI and 52.14% in group CBC) and in the number of neurons/ganglion (33.9% in group CBI and 39.6% in group CBC). The morphological analyses showed that there was an alteration in the shape of the ganglia of the ileal and colonic myenteric plexus. The area of the cell bodies had a significant increase both in the myenteric and the submucous plexus in groups CBI and CBC. These data demonstrate that the BAC treatment causes morphologic and quantitative changes in the myenteric plexus and quantitative changes in the cell body area of the submucous plexus.
Key words: Myenteric plexus. Submucous plexus. Morphology. Benzalkonium chloride. Denervation.
Introduction
The enteric nervous system has been used to study the neuronal plasticity due to its easy access. Besides, it is also used to study the role that nervous plexuses play in their different intestinal functions, as observed in neuronal degenerative diseases (Qualman et al., 1984; Rohrmann et al., 1984; Knusel et al., 1990; Jost and Schimrigk, 1993; Shankle et al., 1993).
A model of intestinal aganglionosis used to study the neuronal plasticity and the myenteric plexus functions is the chemical denervation by serosal application of Benzalkonium chloride (BAC), a cationic surfactant (Sakata et al., 1979; Fox et al., 1983; See et al., 1988, 1990; Zucoloto et al., 1988, 1997; Cracco and Filogamo, 1997; Hernandes et al. 2000). The cytotoxicity of BAC and others detergents in this model are apparently related to their ability to disrupt cell membranes (Fox et al., 1983; Herman and Bass, 1989).
The serosal application of BAC in any segment of the intestine eliminates most of the myenteric neurons (Fox et al., 1983), destroys completely the longitudinal cells and partially the circular cells in the muscle layer (Holle, 1998), without affecting the number of submucosal neurons (Fox et al., 1983; See et al., 1988). It also eliminates the sympathetic innervation of the muscle layer, and mucosal and submucosal plexuses by destroying the extrinsic nerves (See et al., 1990).
This experimental model contributes significantly to the assessment of the role played by the myenteric plexus on several intestinal functions. Although there are other models of intestinal aganglionosis, such as Hirschprungs disease (Howard and Nixon, 1968) and Chagas disease (Köberle, 1963), the denervation model by BAC displays several advantages: (i) in the intrinsic neurons a selective elimination takes place only in the myenteric plexus; (ii) it can be applied to a specific intestinal segment; (iii) it can be performed in genetically intact animals; (iv) it gives the researcher the opportunity to investigate the changes induced by BAC application on several cellular populations (Cracco and Filogamo, 1997).
Within the works performed using this model only a few studied the myenteric plexus influence on the submucous plexus morphology. See et al. (1990) studied only the VIP-immunoreactive neurons of the rat jejunum submucous plexus, and Cracco and Filogamo (1997) the NADH-diaphorase-positive population in the ileum of rats. However, no work has attempted to assess the morphology of the overall neuron population.
The purpose of our work was to observe the denervation effect on the neuronal profile in the submucous and myenteric plexuses of the ileum and colon of rats, by using the immunohistochemistry technique for myosin- V. This technique allows the identification of all the neuronal population (Drengk et al., 2000).
Materials and Methods
Antibodies
Antitail polyclonal antibody, generated against a chicken myosin-V recombinant protein has been characterized previously (Espreafico et al., 1992). A cDNA fragment (corresponding to amino acids 899 to 1830) of a myosin-V-tail clone from a chicken brain was subcloned into pGEX vector to generate a Glutatione transferase (GST)/myosin medial tail (corresponding to amino acids 1117 to 1435) fusion protein in XL1Blue bacteria. This GST/myosin-V medial tail was purified over glutatione resin. This fusion protein was used as an antigen for antibody production in rabbits. The immune serum was purified over a PLM/Myosin-V tail protein affinity column to enrich for IgG antibodies directed against the myosin-V medial tail. Secondary antibodies peroxidase-conjugated Goat antirabbit IgG were used (PIERCE, Rockford, USA).
Experimental design
Animals used in this study were treated in accordance with the guidelines of the Committee on Care and use of Laboratory Animals of the National Research Council. Male Wistar rats weighting about 90 g were anaesthetized with Ketalar® and Rompum®, laparatomized and the proximal colon or ileum brought outside the peritoneal cavity. The proximal colon or ileum were wrapped with cotton soaked in a 2 mM solution of benzyldimethyltetradecylammonium (benzalkonium chloride BAC, Sigma Chemical Co) in saline for 30 min, or with saline only, in the controls. After treatment, the intestinal segment and the entire peritoneal cavity were rinsed with 0.9% saline at 36ºC, followed by closure of the abdomen (Sakata et al., 1979). After surgery, animals were allowed to recover, housed in plastic cages with five animals under controlled temperature conditions and allowed free access to water and laboratory rat chow. The animals were allocated to four groups of five animals each: Group SC in which the colon was treated with saline solution; Group CBC in which the colon was treated with BAC; Group SI, in which the ileum was treated with saline solution; and Group CBI, in which the ileum was treated with BAC.The animals were kept in cages during 15 days and then killed.
Immunohistochemistry of the Myenteric and Submucous Plexuses
The animals were perfused with 1 ml per g body weight of saline solution followed by perfusion with 1 ml/g/body weight of fixation solution containing 10 mM sodium periodate, 75 mM lysine, 1% paraformaldehyde in phosphate buffer 37 mM, pH 7.4 (McLean and Nakane, 1974). Immediately after perfusion, one treated fragment of colon or ileum was removed, flushed with fixative solution, immersed in the same fixative and 15 min later opened and left in this solution for 1 h. Samples were dehydrated through graded alcohols, cleared in xylol, rehydrated back through the ethanol steps to 70% where the tissue could be stored. At this step colon or ileum fragments were dissected separating the muscle wall containing the myenteric plexus from the submucosal layer. The submucosal layer was separated from the mucosal layer by a wooden spatula. Dissection was followed by hydration resumed through 60% and 50% ethanol to PBS. The tissue was washed twice in PBS and blocked for two h with PBS containing 2% BSA, 2% goat serum and 0.5% Triton-X-100 at room temperature.Immuno-staining was proceeded by incubating the tissue fragments in 0.89 µg/ml of affinity-purified antibody specific to the myosin-V medial tail domain diluted in PBS containing 1% BSA, 2% goat serum and 0.1% Triton-X-100, under agitation during 48 h at room temperature. Following incubation, the fragments were washed in PBS containing 0.1% Triton-X-100 and then in PBS with 0.05% Tween-20. They were incubated with 10 µg/ml secondary antibodies conjugated to peroxidase in PBS for 24 h at room temperature under agitation, washed four times for 15 min in PBS containing 0.05% Tween-20. Immunoreaction with peroxidase-conjugated antibodies was developed by incubation with 0.75 mg/ml Diaminebenzidine in PBS and 0.03% H2O2 for 10 min at room temperature under agitation. Samples were mounted in a gel mounting media containing 50% glycerol, 0.07 g/ml gelatin in PBS and 2 µl/ml phenol.
a) Neuron counts: The quantification of the submucous and myenteric plexus neurons was obtained through the counting of cells on the Myosin-V immunohistochemistry stained whole-mount. These whole-mounts had 40 fields randomly selected for counting. An Olympus microscope with 40X objective was used. The total area of the 40 microscopic fields was 6.36 µm2. The results were expressed as number of neurons per mm2.
b) Perikarion area: Neuronal cell profile area measurements were made on 50 randomly chosen cells in each animal (250 neurons per group). For these measurements, images were captured from an Olympus BX50 microscope with 40X objective and processed with an image analysis software (Image-Pro Plus version 4.5.1.22 (2002) of Media Cybernetics, Inc.). The results were expressed in µm2.
Statistical analysis
Students t test was used to compare the number of neurons and neuronal cell profile between each experimental group and its control. Significance level was set at p<0.05.
Results
Qualitative observations of the ganglia and neurons
A regular and uniform pattern was observed in the ileal myenteric plexus of animals in SI group (Fig. 1a). However, there was an irregular distribution in the CBI groups, with regions with no neurons (Fig. 1c). In the ileum, the ganglia shape in the myenteric plexus varied. Elongated, round, triangular, square and rectangular ganglia were observed. Most of the myenteric neurons were elongated, although many round neurons were seen in the ileum.
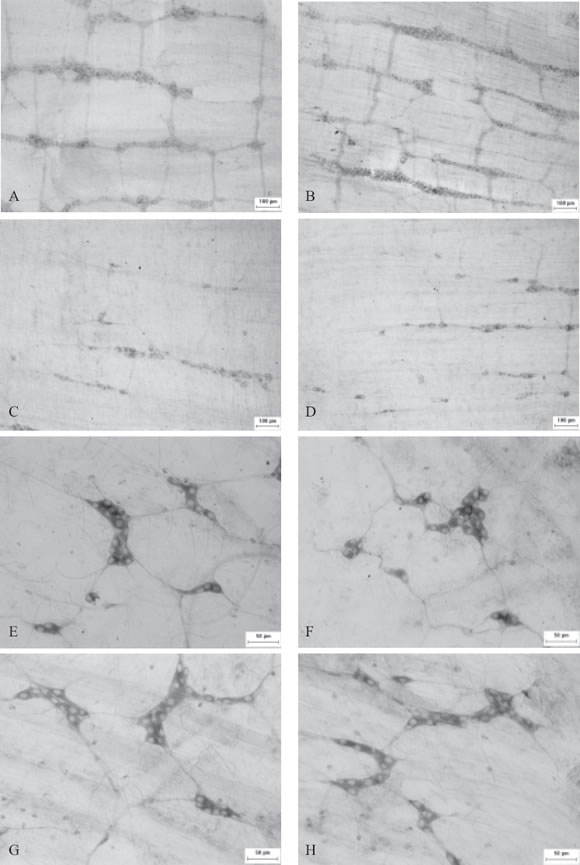
FIGURE 1. Higher magnification of myenteric plexus: SI group (A), SC group (B), CBI group (C), CBC group (D) ganglia and fibres with the myosin-V medial tail antibody and submucous plexus: SI group (E), SC group (F), CBI group (G) and CBC group (H).
In the submucous plexus, the ganglia in the SI and CBI groups were nodular and showed a regular pattern of distribution (Fig. 1b, 1d). The ganglia were polygonal or triangular; some were elongated in shape and distinctly separated. Neurons were generally smaller, with elongated, roundish or pear-like profiles. Few neurons were as elongated as those in the myenteric plexus.
In the proximal colon, we observed that the myenteric plexus had the same pattern as in the ileum. In the SC group, the pattern of distribution was regular and uniform (Fig. 1b). The CBC group showed an irregular distribution, with absence of neurons in some areas (Fig. 1d). The neurons had the same morphology as those in the ileal myenteric plexus.
We noticed a different distribution pattern in the colonic submucous plexus, with ganglion accumulation in colonic folds both in the SC and CBC groups. The morphology of the colonic submucous plexus neurons was identical to the ileal submucous plexus neurons.
Neuron size
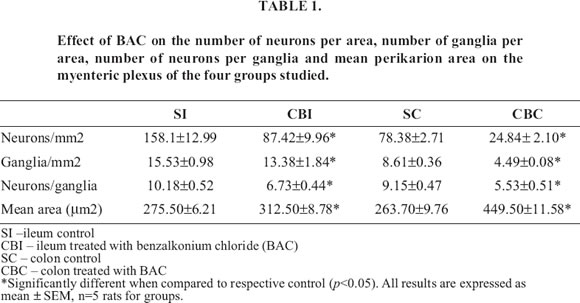
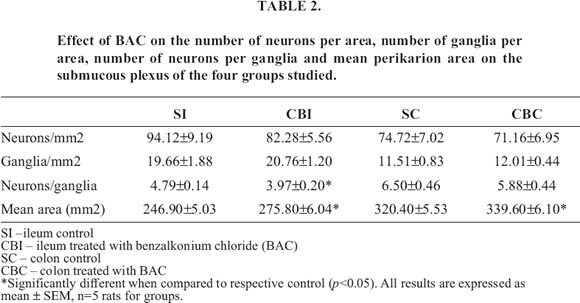
The ranges of perikaryon size, expressed as perikaryon area in all plexuses examined, are shown in Table 1 and 2. All the analyzed areas presented significant differences when compared with their respective controls.
Neurons counts
All the analyzed parameters in the myenteric plexus of ileum and colon showed significant differences (Table 1).
There was a significant difference in the number of neurons per ganglia in the ileal submucous plexus (Table 2). As for the colonic submucous plexus, no significant differences in any of the analyzed parameters were seen (Table 2).
Discussion
Few quantitative and morphometric studies on the myenteric and submucous plexuses have been done employing the BAC denervation model. Most of the works deal only with the degree of denervation in the myenteric plexus after BAC treatment (Sakata et al., 1979; Fox et al., 1983; See et al., 1988, 1990; Zucoloto et al., 1988, 1997; Cracco and Filogamo, 1997; Hernandes et al. 2000) or with specific populations of the submucous plexus neurons (Fox et al., 1983; See et al., 1990; Cracco and Filogamo, 1997).
The myenteric plexus morphology found in the ileum and colon show elongated ganglia parallel to the circular muscle, as mentioned by other authors (Gabella, 1987). The ganglion shape varies among species and they may be elongated, geometrical (polyedric) or roundshaped (Gabella, 1987). We found elongated, triangular and polygonal ganglia in the SI group, similar to Hernandes (1994) findings in the ileum of rats. The nervous cell distribution pattern was very different in the CBI and CBC groups when compared with the SI and SC. Extensive areas devoid of myenteric innervation could be observed, while others display isolated, spindle-shaped ganglia made up of few neurons, organized in irregular networks. The neurons presented a spherical, pear-like or fusiform shape in the ileum and colon. The ganglia in the ileum were closely packed, whereas they were more widely spaced in the colon.
The submucous plexus ganglia were nodular and showed a regular pattern of distribution in the ileum while the submucous plexus in the colon showed ganglia with more neurons in pleat region. The neurons were mainly spherical, elongated or pear-like in shape in the ileum and colon as observed by Liberti et al. (1994) in the small intestine of guinea pigs.
The technique employed allowed us to observe that the ganglia are connected through bundles of nervous fibers (Fig. 1).
The BAC treatment caused an expressive increase in the perikaryon area of both the myenteric plexus cells and the submucous plexus cells (Table 1,2). Cracco and Filogamo (1997), using the NADH-diaphorase histochemistry in the rat ileum, observed a reduction in the perikaryon area in denervated animals (460 ± 110 µm2 in controls; 285 ± 94 µm2 in denervated animals).
These results might be due to the technique employed, which stains only the neuronal population enzimatically active (Young et al., 1993). There was also a significant increase in the perikaryon area in the submucous plexus in the denervated group, whit the SC showing a mean area of 320.4 ± 5.52 µm2 and the CBC group 339.6 ± 6.10 µm2. Barbosa (1973) studied the correlation between the increase in the cytoplasmic area and the smaller number of nervous cells, making a comparison between the colon and the cecum, knowing that the cecum has less neurons per area than the colon. See et al. (1990), when studying the VIP-immunoreactive neurons, observed an increase of 2.5% in the perikaryon area 15 days after the denervation. Dahl et al. (1987) observed that there was a compensatory increase in the production of certain neurotransmitters by the surviving neuronal elements. This increase in the cell area of myenteric and submucous plexus neurons in the ileum and colon may be due to a compensatory neuronal hypertrophy, with the purpose of increasing neurotransmitter production, since the number of myenteric plexus neurons in these two segments decreased (Table 1,2).Other works (See et al., 1988, 1990; Zucoloto et al., 1988, 1997; Holle et al., 1998) showed that the chemical denervation with BAC causes a hypertrophy in the circular and longitudinal muscle resulting an increase in the neurons cell profile area due to an increase in the area to be innervated by them. After inducing muscle hypertrophy in the guinea pig ileum, Gabella (1990) noticed an increase in the perikarion area in the myenteric and submucous plexus of these animals. Studies have proved that chemical ablation with BAC causes the denervation of the circular muscle soon after the treatment. However, 30 days later there is a re-innervation by neurons of the submucous plexus (Herman and Bass, 1990). Cracco and Filogamo (1997), using the NADH-diaphorase histochemistry, demonstrated a significant increase in the perikaryon area in the submucous plexus of the BAC-treated-group. These authors suggest that the submucosal neurons hypertrophy in the BAC-treated segment may be caused by the lack of inhibitory influence exerted by the neural input (both myenteric and extrinsic) on their growth and by the increased mass of the target tissue innervated (Gabella, 1990), i.e. increased volume of the mucosa (See et al., 1990; Hernandes et al. 2000) and partial re-innervation of the thickened circular muscle layer (Luck et al. 1993). See et al. (1990) also describe enlarged cell bodies of the VIP-immunoreactive neurons of the submucosal plexus.
Quantitative parameters of neurons in the myenteric and submucous plexuses were thoroughly studied in this work. Here, we studied the number of neurons per area, the number of ganglia per area and the number of neurons per ganglia. No other work using the BAC chemical denervation model has analyzed these last two parameters. A significant reduction was only found in the myenteric plexus when the number of neurons/mm2 was analyzed, as reported by other authors (Sakata et al., 1979; Fox et al., 1983; See et al., 1988, 1990; Zucoloto et al., 1988, 1997; Cracco and Filogamo, 1997; Hernandes et al. 2000; Dahl et al., 1987; Herman and Bass, 1989, 1990; Luck et al., 1993). In our experiment, the denervation of the proximal colon after 15 days treatment was 68.31% and 55.29% in the ileum. Our results were similar to those found by Buttow et al. (2003), who found a denervation degree of 69% in the proximal colon when using the immunohistochemistry technique for myosin-V in animals of the same age.
There is a large variation on the denervation percentage in experiments using BAC; this may be a consequence of the animal age and also of the dose given at the beginning of the experiment (Hernandes et al., 2000; Herman and Bass, 1989). Garcia et al. (2002), studying the influence of aging on the number of neurons of the myenteric plexus, observed that the animals treated with BAC after 2, 6, 12 and 18 months, showed a greater neuronal survival when compared to controls, and suggest that these results are due to a neuroplasticity phenomenon. The colon denervation of young rats, weighing about 60 g, reached 78% of neurons of the myenteric plexus five months after 0.2% BAC treatment (Zucoloto et al., 1988). Zucoloto et al. (1997), induced the myenteric plexus ablation with 2 mM BAC in the distal colon of young rats weighting about 50 g, and presented a reduction around 57% in the number of neurons of the plexus, ten days after BAC treatment.
As it was demonstrated in other works, there was no significant reduction in the number of submucous plexus neurons (Fox et al., 1983; See et al., 1988, 1990; Cracco and Filogamo, 1997). According to Fox et al. (1983), for an unknown reason, the diffusibility of BAC through the circular muscle was hindered. It is also possible that the myenteric neurons are more sensitive to BAC than the submucosal neurons.
By analyzing the number of ganglia/mm2, a significant reduction only in the ileal and colonic myenteric plexuses was observed. We found a significant reduction in the ileum and colon myenteric plexus, and in the ileum submucous plexus by observing the number of neurons per ganglia. The alterations seen in the myenteric plexus are the result of a reduction in the number of neurons; there is also a decrease in the number of ganglia per unit area and in the number of neurons per ganglion. No significant reduction in the number of ganglia per millimeter in the submucous plexus was observed; however, there was a significant reduction in the number of neurons per ganglion in the ileum of the CBI group. This may be a consequence of a small neuron dilution caused by the small dilatation seen in the treated segment.
This work allowed us to conclude that the serosal treatment with BAC in the ileum and colon causes a significant change in the quantitative parameters analyzed, especially in the myenteric plexus of the ileum, and that the dilatation seen in the treated segment may alter some parameter in the submucous plexus, with no denervation taking place. There were no morphological alterations in the neurons and ganglia shape in the submucous plexus when compared with the controls; however, the myenteric plexus ganglia showed an irregular pattern due to denervation. There was also a significant increase in the area of these cells as a result of the treatment in the myenteric as well as the submucous plexus.
Acknowledgemensts
The authors would like to thank Dr Enilza Maria Espreafico (USP – Ribeirão Preto/Brazil) for her invaluable assistance and support in the production of the anti myosin-V antibody.
References
Barbosa AJA (1973). Auerbachs plexus of the albino rat. I. Quantitative study of the ganglia and nerve cells in the caecum and colon. Pesquisas Med Biol 6: 253-62. [ Links ]
Büttow NC, Zucoloto S, Espreafico EM, Gama P, Alvares EP (2003). Substance P enhances neuronal area and epithelial cell proliferation after colon denervation in rats. Dig Dis Sci. 48: 2069-2076. [ Links ]
Cracco C, Filogamo G (1997). Neuronal and non-neuronal plasticity in the rat following myenteric denervation. Adv Exp Med Biol 429:159-169. [ Links ]
Dahl JL, Bloom DD, Epstein ML, Fox DA, Bass P (1987). Effect of chemical ablation of myenteric neurons on neurotransmitter levels in the rat jejunum. Gastroenterology 92: 338-344. [ Links ]
Drengk AC, Kajiwara JK, Garcia SB, Carmo VS, Zucoloto S, Larson RE, Espreafico EM (2000). Imunolocalization of myosin-V in the enteric nervous system of the rat. J Auton Nerv Syst 78: 109-112. [ Links ]
Espreafico EM, Cheney RE, Matteoli M, Nascimento AAC, de Camilli P, Larson RE, Mooseker MS (1992). Primary Structure and cellular localization of Chicken brain Myosin-V (p190), an unconventional Myosin with calmodulin light chains. J Cell Biol 119:1541-1557. [ Links ]
Fox DA, Epstein ML, Bass P (1983). Surfactants selectively ablate enteric neurons of the rat jejunum. J Pharmacol Exp Ther 227: 538-544. [ Links ]
Gabella G (1987). The number of neurons in the small intestine of mice, guinea-pigs and sheep. Neuroscience 22: 737-752. [ Links ]
Gabella G (1990). Hypertrophic smooth muscle. V. Collagen and other extracellular materials. Vascularization. Cell Tissue Res 235: 275-283. [ Links ]
Garcia SB, Demarzo MM, Vinhadeli WS, Llorach-Velludo MA, Zoteli J, Herrero CF, Zucoloto S (2002). No reduction with ageing of the number of myenteric neurons in benzalkonium chloride treated rats. Neurosci Lett 331: 66-68. [ Links ]
Herman JR, Bass P (1989). Enteric Neuronal Ablation: Structure-Activity Relationship in a Series of Alkyldimethylbenzylammonium Clorides. Fundam Appl Toxicol 13: 576-584. [ Links ]
Herman JR, Bass P (1990). Pharmacologic characterization of the changes in cholinergic sensitivity of rat jejunal circular muscle after myenteric plexus ablation. J Pharmacol Exp Ther 252: 135-139. [ Links ]
Hernandes L (1994). Estudo morfológico da mucosa e do corpo celular dos neurônios do plexo mientérico do íleo de ratos com diabetes mellitus induzido por estreptozootocina. Maringá PR Brazil. These to presented in the Universidade Estadual de Maringá 120 p. [ Links ]
Hernandes L, Zucoloto S, Parisi EP (2000). Effect of myenteric denervation on intestinal epithelium proliferation and migration of suckling and weanling rats. Cell Prolif 33: 127-138. [ Links ]
Holle GE (1998). Changes in muscularis externa of rat small intestine after myenteric ablation with Benzalkonium Chloride. Dig Dis Sci 43: 2666-2675. [ Links ]
Howard ER, Nixon HH (1968). Internal anal sphincter. Observations on development and mechanism of inhibitory responses in premature infants and children with Hirschprungs disease. Arch Dis Child 43: 569-578. [ Links ]
Jost WH, Schimrigk K (1993). Cisapride treatment of constipation in Parkinsons disease. Mov Disord 8: 339-343. [ Links ]
Knusel B, Jenden DJ, Lauretz SD, Booth RA, Rice KM, Roch M, Waite JJ (1990). Global in vivo replacement of choline by N-aminodeanol. Testing a hypothesis about progressive degenerative dementia: I. Dynamics of cholina replacement. Pharmacol Biochem Behav 37:799-809. [ Links ]
Köberle F (1963). Enteromegaly and Cardiomegaly in Chagas disease. Gut 4: 399-405. [ Links ]
Liberti EA, Queiroz LM, Popeu E, Perito MAM, Minarelli AM, Moraes JOR, Souza RR (1994). A quantitative and comparative study of the ganglionic neurons in the myenteric and submucous plexuses of the small intestine, and in the intramural plexus of the fall bladder of the guinea-pig. Rev Bras Cien Morfol 11:106-114. [ Links ]
Luck MS, Dahl, JL, Boyeson MG, Bass P (1993). Neuroplasticity in the smooth muscle of the myenterically and extrisically denervated rat jejunum. Cell Tissue Res 271: 363-374. [ Links ]
McLean IW, Nakane PK (1974). Periodate-Lysine-Paraformaldehyde Fixative. A New Fixative for Immunoeletron Microscopy. J Histochem Cytochem 22: 1077-1083. [ Links ]
Qualman SJ, Haupt HM, Yang P, Hamilton SR (1984). Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinsons disease. Gastroenterology 87: 848-856. [ Links ]
Rohrmann CAJr, Ricci MT, Krishnamurthy S, Schuffler MD (1984). Radiologic and histologic differentiation of neuromuscular disorders of the gastrintestinal tract: visceral myopathies, visceral neuropathies and progressive systemic sclerosis. Am J Roentgenol 143: 933-941. [ Links ]
Sakata K, Luneida T, Furuta T, Sato A (1979). Seletive destruction of intestinal nervous elements by local application of benzalkonium solution in the rat. Experientia 35: 1611-1613. [ Links ]
See NA, Epstein LE, Schultz E, Pienkowski TP, Bass P (1988). Hyperplasia of jejunal smooth muscle in the myenterically denervated rat. Cell Tissue Res 253: 609-617. [ Links ]
See NA, Epstein LE, Dahl JL, Bass P (1990). The myenteric plexus regulates cell growth in rat jejunum. J Auton Nerv Syst 31: 219-230. [ Links ]
Shankle WR, Landing BH, Ang SM, Chui H, Villarreal-Engelhardt G, Zarow C (1993). Studies of the enteric nervous system in Alzheimer disease and other dementias or the elderly: enteric neurons in Alzheimer disease. Mod Pathol 6: 10-14. [ Links ]
Young HM, Furness JB, Sewell P, Burcherm EF, Kandiah CJ (1993). Total numbers of neurons in myenteric ganglia of the guinea-pig small intestine. Cell Tissue Res 272: 197-200. [ Links ]
Zucoloto S, Diaz JA, Oliveira JSM, Muccilo G, Sales-Neto V, Kajiwara JK (1988). Effect of chemical ablation of myenteric neurons on intestinal cell proliferation. Cell Tissue Kinet 21: 213-219. [ Links ]
Zucoloto S, Deus DA, Martins AA, Muglia VF, Kajiwara JK, Garcia SB (1997). The relationship between myenteric neuronal denervation, smooth muscle thickening and epithelial cell proliferation in the rat colon. Res Exp Med 197: 117-124. [ Links ]
Received on May 28, 2003.
Accepted on February 9, 2004.














