Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.28 no.2 Mendoza Apr./Aug. 2004
Probiotics enhance the recovery of gut atrophy in experimental malnutrition
Diana B. Dock1, José E. Aguilar-Nascimento2 and Marcia Q. Latorraca1
1 Department of Nutrition & Dietetics, Nursing and Nutrition School, The Federal University of Mato Grosso, Brazil
2 Department of Surgery, The Federal University of Mato Grosso, Brazil.
Address correspondence to: Prof. José Eduardo de Aguilar-Nascimento. Rua Estevão de Mendonça 81 apto 801, CEP 78045-200 Cuiabá – MT, BRASIL. Tel: + 55 65 6237183; Fax: + 55 65 6247149; E-mail: aguilar@terra.com.br
Abstract: AIM: The aim of this study was to evaluate the effect of probiotics on the recovery of the bowel atrophy induced by malnutrition in rats. METHODS: Twenty-and-six Wistar rats (200-250g) were fed with either a normoproteic (sham group, n=6) or a free-protein diet (n=20) during 12 days. Twelve malnourished rats were randomized to recover during 15 days with either a hydrolyzed diet (control group, n=6) or the same diet enriched with probiotics (Streptococcus thermophilus and Lactobacillus helveticus; probiotic group, n=6). RESULTS: Probiotic group showed similar gain of body, liver and bowel weight than controls. At the jejunum, both the villus height (383±49 vs. 321±46mm; p=0.04) and crypt depth (157±31 vs. 125±10mm; p=0.04) were greater in probiotic group than in controls. The crypt depth at the cecum (214±22 vs. 169±43 mm; p=0.05) and the wall width at both the cecum (410±18 vs. 340±61 mm; p=0.02) and sigmoid (479±130 vs. 330±62 mm; p=0.03) were higher in probiotic than in control group. CONCLUSION: Streptococcus thermophilus and Lactobacillus helveticus enhance the recovery of gut atrophy induced by malnutrition. Probiotics can be useful as oral adjuvants during the recovery of malnutrition.
Key words: Probiotics. Malnutrition. Small bowel. Colon. Mucosa.
Introduction
The human gastrointestinal tract has long been considered as simply being to digest and absorb nutrients and excrete waste end products. However, the important role of the gut mucosal barrier to avoid the entrance of microorganisms and toxins has been now largely accepted (McNaught and MacFie, 2001). The gut flora is one of the main constituents of this defense barrier and is considered the first line of defense of the gut. In fact, failure in this mechanism, results in an increased antigen and toxin transport across the gut mucosa (Allori et al., 2000).
The intestine is the main immunological organ: it contains 50% of all reticulo endothelial and other immune cells, and produces the greatest amount of secretory IgA (Hulsewe et al., 1999). The gut-associated lymphoid tissue (GALT) represents the largest mass of lymphoid tissue in the human body (Isolauri et al., 2001).The stimulation of host immunity is related to the ability of microorganisms to adhere to the mucosa and interact with the GALT (McGhee et al., 1992). The ability of some microorganisms to adhere to the intestinal cells may difficult pathogenic bacterial colonization and thus, contributes to diminish bacterial translocation. In this context, probiotics, currently defined as live microflora feed supplement that beneficially affects the host animal by improving its intestinal microbial balance (Fuller, 1989), may enable valuable modifications of the immune system (Fuller, 1989; Isolauri, 2001).
Protein malnutrition disrupts the normal ecology of the microflora affecting strictly anaerobes (Tannock and Savage, 1974; Poxton et al., 1997), impairs host immune response and antibacterial defenses (Reynolds et al., 1992; Chandra, 1993), enhances the susceptibility to infection, and leads to mucosal atrophy (Reynolds et al., 1996). Malnutrition is a common problem for critical ill patients and nutritional support is mandatory. During renutrition process, both the composition of the diet and the administration route have a profound influence on intestinal morphology and function (Allori et al., 2000). Hydrolyzed diets, including those containing hydrolyzed proteins as nitrogen source, are frequently used to recovery malnourished patients (Silk, 1987; Meredith et al., 1990). Tripeptides and dipeptides are more efficiently utilized than free amino acids, have greater nutritional value, are better absorbed, and retain more nitrogen than intact protein, contributing to enhance the gain of weight (Silk, 1987; Zaloga and Prielipp, 1991). However, these diets dispense few substrates for the colonic microorganisms and thus, may impair the gut microflora balance and immune response. Some immunonutrients, especially glutamine, arginine and omega-3 fatty acids have been associated with enteral feeding to enhance the immune response (Daly et al., 1992). Lactic acid bacteria or fermented milk added to an enteral feeding formula would improve not only the nutritional state but also the intestinal microflora and the immune response (Isolauri et al., 1991; Perdigon and Alvarez, 1992; Perdigon et al., 1995).
Few studies have investigated the role of probiotics on the intestinal trophism after malnutrition (Ichikawa et al., 1999; Allori et al., 2000). Recently, it was shown that probiotics might induce the formation of short chain fatty acids (SCFA) and thus contribute to the colonic trophism (Sakata et al., 1999). Considering that the intestinal atrophy due to malnutrition is rapidly reverted with a proteic supplement, it could be hypothesized that the implement of probiotics on the offered diet may enhance even more the recovery of the atrophic gut. Therefore, an experimental study focusing on the relationship between probiotic bacteria and the intestinal trophism during renutrition could be interesting. Thus, the aim of this present study was to investigate the effects of the addition of probiotic bacteria to a hydrolyzed diet on the recovery of the intestinal morphology during renutrition in an animal model of malnutrition.
Materials and Methods
The experiment follows the COBEA (Brazilian Committee on Experimental Animal Care) adopted by the Federal University of Mato Grosso. Twenty-six male Wistar rats (200-250g) were included in the study. They were kept in a laboratory environment of light/dark cycles for three days prior to the experiment. All animals had free access to water. Animals received either a free-protein diet (Rhoster São Paulo, Brazil; composition per 100g: 0.55g protein, 88.2g carbohydrate, 7.0g lipid, in addition to minerals and vitamins; aproteic group, n=20) or a standard rat chow (Rhoster AIN-93, São Paulo, Brazil; composition per 100g: 17.5g protein, 67.9g carbohydrate, 7.0g lipid, in addition to minerals and vitamins; sham group, n=6) for 12 days. Induction of malnutrition after 12 days with this diet was published earlier (Dock et al., 2003).
A group of eight animals of the aproteic group and all animals of the sham group were killed after collection of blood samples for laboratory analysis on the 13th day. Analyses were performed at Central Laboratory of Julio Muller Universitary Hospital. During necropsy, the spleen and the liver were ressected and weighted. The entire small and large bowel were dissected, freed from the mesentery, and weighted after the contents were gently removed. The length of both intestines was registered as described earlier (Aguilar-Nascimento et al., 1999). Two cm long full thickness biopsies from the jejunum (one cm below the Treitz angle), ileum (two cm above the ileocecal valve), cecum (one cm distal to the ileocecal valve) and sigmoid (two cm above the peritoneal reflexion) were collected and sent for histological analysis in 10% formalin. Slides containing three or four histological sections of 4 µmthick cut sagitally to the mucosa were stained with hematoxylin and eosin, and examined by a experienced blind examiner with an optical microscope (100 X magnification). The crypt depth, villus height and wall width in each slide were obtained using a micrometer ruler (Olympus, Japan). The mean of the ten best-oriented glands of each intestinal site was considered to represent the data for each specimen as published earlier (Aguilar- Nascimento et al., 1999).
The rest of the aproteic group (n=12) were randomized to two groups to recover from malnutrition by receiving either a hydrolyzed diet (20 g/day) (composition per 100g: 19.96 g protein [80% dipeptides and 20% aminoacids], 64.47 g carbohydrate [88% maltodextrin and 12% starch], 15.57 g lipid [50% mid-chain triglycerids, 30% milk lipids and 20% corn oil]; control group, n=5) or the same diet (16 g/day) plus reconstituted milk (4.0 g/day) containing 106 cfu/g of Streptococcus thermophilus e Lactobacillus helveticus (Bionan, Nestlé, Brazil) (final composition per 100 g: 18.60 g protein, 62.15 g carbohydrate, 19.25 g lipid, probiotic group, n=6) for 15 days. The two diets were isoenergetic and isonitrogenous. The amount of food consumed was registered daily and the caloric intake calculated. The animals weight was obtained each four days. On the 16th day, blood samples were collected and the animals were killed. Again, the same procedure for morphological study above described was done. Laboratory analysis in all phases included serum total proteins, globulin and albumin.
Independent Students t test or Mann-Whitney test was used to compare continuous variables between two groups in each phase depending on the homogeneity of the data (Levenes test). Two-way ANOVA for repeated measures followed by HSD Tukeys test was used to compare the weight gain and the food intake during the evolution of the experiment between the two groups. One-way ANOVA or Kruskal-Wallis test followed by Tukeys test was used to compare control and probiotics groups with aproteic group when necessary. As most analysis was performed by parametric tests and only in three occasions (comparisons of crypt depth at the jejunum and wall width at both jejunum and cecum) nonparametric tests were used, all results are presented as mean ± SD. A 5% level was established as being statistically significant. Analyses were done using the statistical package "Statistic for Windows" (Stat Soft, Inc., Tulsa, OK, USA).
Results
Malnutrition phase
Protein restriction had a significant effect on food intake during the 12 days of the experimental period. It was observed a significant decrease in food intake overtime only in animals of aproteic group (Fig. 1A).
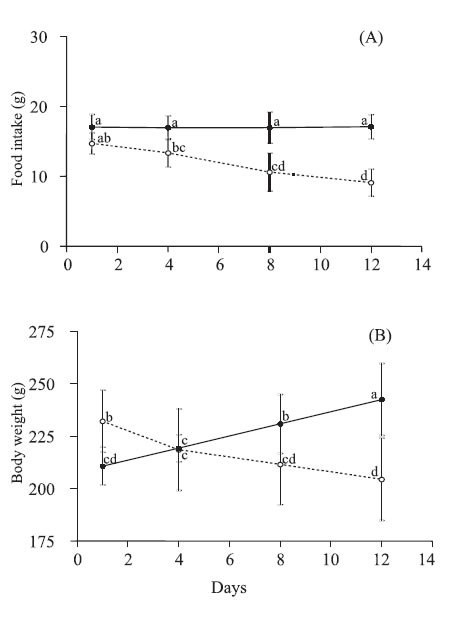
FIGURE 1. Food intake (A) and body weight (B) of rats fed on a standard rat show (sham group; solid lines) or aproteic (aproteic group; dotted lines) diet during 12 days. Values are mean with their standard deviation represented by vertical bars for 6 and 8 rats from sham and aproteic groups, respectively. Different letters indicate significant differences between groups and time (Tukey HSD, p<0,05).
Mean body weight of aproteic group was higher than that of sham group at the beginning of the malnutrition phase (232 ± 15 g vs. 211 ± 9 g, p<0.05). On the 4th day, the weight of rats of aproteic group reduced 6%, whereas the sham group increased 4% in relation to their initial values. This pattern persisted during all the malnutrition phase. On the 12th day, the mean weight of animals of the aproteic group was reduced 84% when compared to that observed in the sham group (Fig. 1B).
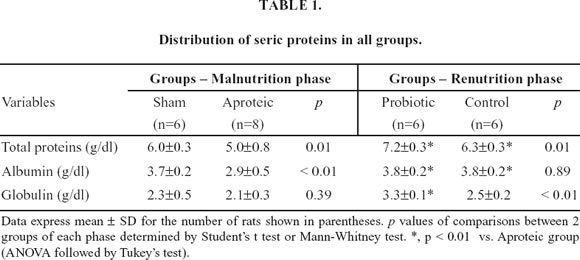
In addition to reduction of body weight, the aproteic group manifested other typical features of protein malnutrition, including diminished organ weight, hypoalbuminemia and low-serum total protein when compared with the sham group. However, no difference occurred in globulin levels when the two groups were compared (Table 1).
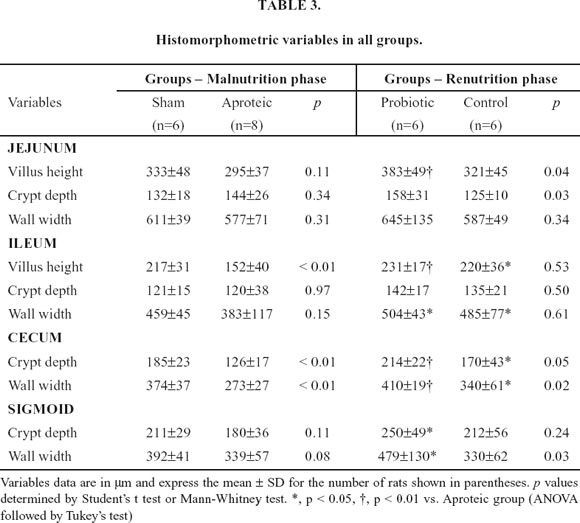
At both the jejunum and the sigmoid, protein restriction did not promote any significant differences in all the histological parameters. However, at the ileum villus height was significantly shorter in malnourished animals (152±40 vs. 217±31 µm; p <0.01) and at the cecum both the crypt depth (126±17 vs. 185±23 µm; p<0.01) and the wall width (273±27 vs. 374±37 µm; p<0.01) were reduced in aproteic animals (Table 3).
Renutrition phase
During renutrition phase, food intake was similar in animals of probiotic and control groups over time (Fig. 2A). However, at both the 3rd and the 15th day of this phase, food intake of rats receiving probiotics was lower than their initial values (Fig. 2A).
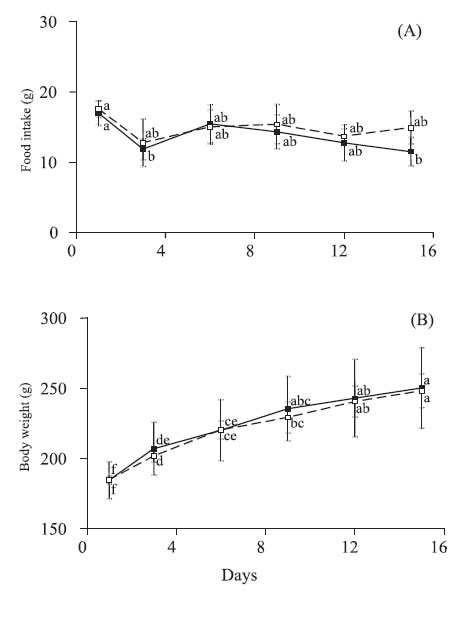
FIGURE 2. Food intake (A) and body weight (B) of rats fed on a hidrolized diet (control group; dotted lines) or hydrolyzed diet plus S. thermophilus and L. helveticus diet (probiotic group; solid lines) during 15 days. Values are means with their standard deviation represented by vertical bars for 6 rats in both control and probiotic groups. Different letters indicate significant differences between groups and time (Tukey HSD, p<0,05).
At the beginning of the renutrition phase, probiotic and control rats had similar body weight. In both groups, the rats maintained a steady gain of body weight. This resulted in mean values of 35% and 34% higher than the initial body weight for probiotic and control groups, respectively (Fig. 2B). The feed efficiency, calculated as the ratio of weight gain (g) per gram of food intake over 15 days did not differ between the probiotic and control animals.
Compared to aproteic rats, both renutrition groups significantly increased seric protein and albumin. However, probiotic group showed greater serum globulin than both aproteic and hydrolyzed groups (Table 1).
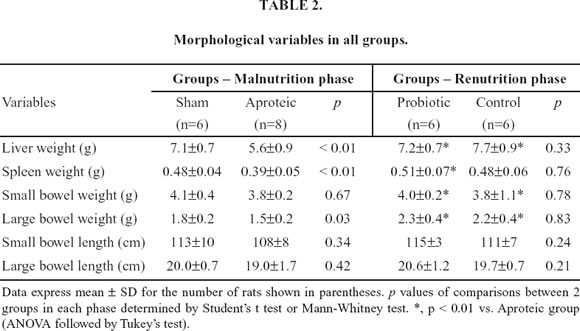
Table 2 shows the findings of the morphological variables in all groups. Renutrition with either hydrolyzed or hydrolyzed plus probiotics promoted significant increase in the liver, small bowel and large bowel weights when compared with aproteic animals. However, only rats recovered with probiotic diet presented significant gain of spleen weight when compared with malnourished rats. The length of both the small and large bowels did not differ among groups.
Comparison of histological data between renourished groups showed significant difference favoring probiotic group especially at the large bowel (Table 3). At the jejunum, both the villus height (383±49 vs. 321±46 µm; p=0.04) and crypt depth (158±31 vs. 125±10 µm; p=0.03) were greater in probiotic group than in control group. Although no difference occurred at the ileum between the animals fed with the tworenutrition diets, both groups showed significant greater villus height and wall width than aproteic animals. The crypt depth at the cecum (214±22 vs. 170±43 µm; p=0.05) and the wall width at both the cecum (410±19 vs. 340±61 µm; p=0.02) and sigmoid (479±130 vs. 330±62 µm; p=0.03) were higher in animals fed with probiotics than in control group. Compared with aproteic group, probiotic fed rats presented significant higher values in at least one measure in all bowel segments. Only probiotic group presented significant greater values than aproteic group in the following measures: villus height at the jejunum and both crypt depth and wall width at the sigmoid.
Discussion
Protein malnutrition enhances susceptibility to infections due predominantly to impaired systemic immune function (Windsor and Hill, 1988). In this study, protein restricted rats spontaneously reduced their food intake, showed significant loss in body, liver, spleen and colonic weight, and coursed with hypoproteinemia and hypoalbuminemia. During renutrition phase, both groups similarly presented improvement in feed efficiency, gain of body, liver, small bowel and colonic weight, and in albumin levels. As both renutrition diets were isocaloric and isonitrogenous these findings were expected, and already documented in another study (Allori et al., 2000).
The results also showed that the use of probiotics was associated with greater gain of spleen weight and with higher levels of total seric protein due to increased globulin levels. Experimentally, other authors have substantially documented an augment of the level of seric immunoglobulin associated with probiotics (Puri et al., 1994; Dock et al., 2003). The nutritional benefits of lactic acid bacteria in fermented dairy products have been well documented, especially in terms of weight gain, feed efficiency (Thoreaux et al., 1998) and stimulation of the immunity (Fuller, 1991). Although higher concentration of seric globulin does not necessarily reflect greater production of antibodies, some studies have documented an increase of seric IgA after ingestion of probiotics (Link-Amster et al., 1994; Puri et al., 1994). Secretory IgA, produced by B intestinal cells may cross the mucosal barrier and enter the blood stream influencing the rise of seric IgA (Puri et al., 1994). Thus, the higher level of globulin and the greater spleen weight suggest an increase production of seric immunoglobulin influenced by probiotic diet. However, this is most speculative and necessarily has to be proved in further investigations.
The overall results of the histomorphometric study consistently demonstrated a faster recovery from the gut atrophy status associated with addition of Streptococcus thermophilus and Lactobacillus helveticus in the diet. Although the hydrolyzed diet alone had improved some histomorphometric variables than before renutrition, the best results were seen in probiotic group. Moreover, when the two-renutrition groups were compared, the probiotic diet was associated with greater mucosa width (villus height and crypt depth) at both the jejunum and the cecum, and with greater colonic wall width at both the colonic sites. The enhanced mucosal trophism found in this experiment with the addition of Streptococcus thermophilus and Lactobacillus helveticus in the renutrition diet was also reported in studies involving other probiotic bacterias (Ichikawa et al., 1999; Allori et al., 2000). These results are most probably due to enhanced SCFA formation induced by probiotics (Sakata et al., 1999). SCFA is the best fuel for the colonocyte and directly trophic for the colonic mucosa (Roediger, 1980). The findings of best recovery of atrophy at the colon were probably due to SCFA production.
Under conditions of malnutrition the intestinal flora is affected, the gut mucosal barrier is impaired and thus, translocation may occur (Deitch et al., 1987). Therefore, the potential benefit of rapidly improve the mucosal barrier is to prevent bacterial translocation. In this context, probiotic bacteria may prevent bacterial translocation in some animal models (Tuomola et al., 1999; Garcia-Urkia et al., 2002). Probiotics may assist the recovery of malnutrition augmenting the resistance to colonization by enteric pathogens, by inducing the recruitment of lamina propria immune cells, by competing more successfully for essential enteric nutrients, and by metabolizing non-absorbable nutrients into volatile fatty acids and chemically modified bile acids (Lu and Walker, 2001; Dock et al., 2003). Other potential benefit of including probiotics as a supplement of renutrition diets is to restore the normal microflora (Allori et al., 2000). Moreover, in an animal model of colitis, probiotic bacteria enhanced not only the mucosal barrier but also improved the histological disease, and diminished the mucosal secretion of TNF α and interferon δ (Madsen et al., 2001). Put all together, all these effects verified in experimental studies suggest that probiotics improve gut defenses and at the same time decrease the inflammatory response. However, in the clinical setting the use of probiotics has been reported as controversial (MacNaught and MacFie, 2001).
The trophic effect in the small bowel associated with probiotics was most evident at the jejunum. We could not find a reasonable explication for this finding associated with the use of Streptococcus thermophilus and Lactobacillus helveticus. However, other study investigating the recovery of malnutrition with two different bacteria showed that the morphological response at either the jejunum or the ileum was different with each bacteria involved. The findings observed in both clinical and experimental studies with probiotics have shown that the results are influenced by the type and concentration of the probiotic bacteria used (Isolauri et al., 2001), by the length of time of the treatment (Perdigon et al., 1990; Perdigon et al., 1991), by the age (Allori et al., 2000), and clinical conditions of the host (Perdigon et al., 1990). Therefore, conclusions on this issue should be taken cautiously and further studies on this field are largely expected.
The overall results of this study showed that the addition of the two-probiotic bacteria positively influenced a more rapid restoration of the gut atrophy associated with malnutrition. Although the findings of an experimental study should be transposed to the clinical setting with caution it could be concluded that Streptococcus thermophilus and Lactobacillus helveticus added to a renutrition diet enhance the recovery of the gut atrophy induced by malnutrition. Immediate clinical application of this would be the improvement of the gut mucosal barrier.
Acknowledgements
The authors wish to thank Mr. Celso Roberto Afonso for his technical assistance. This work was supported by the Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT). Supported by Fapemat, Grant 2000/01.
References
Aguilar-Nascimento JE, França da Silva LR, de Oliveira AF, Gomes da Silva MH (1999). Enhanced mucosal re-epithelialization induced by short chain fatty acids in experimental colitis. Brazilian Journal of Medical and Biological Research 32: 961-966. [ Links ]
Allori C, Aguero G, Ruiz HAP, Nader OM, Perdigon G (2000). Gut mucosa morphology and microflora changes in malnourished mice after renutrition with milk and administration of Lactobacillus casei. Journal of Food Protection 63: 83-90. [ Links ]
Chandra RK (1993). Nutrition and the immune system. The Proceedings of the Nutrition Society 52: 77-84. [ Links ]
Daly JM, Lieberman MD, Goldfine J, Shou J, Weintraub F, Rosato EF, Lavin P (1992). Enteral nutrition with supplemented arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic and clinical outcome. Surgery 112: 56-67. [ Links ]
Deitch EA, Winterton J, Li M, Berg R (1987). The gut as portal of entry for bacteremia role of protein malnutrition. Annals of Surgery 205: 681-692. [ Links ]
Dock DB, Aguilar-Nascimento JE, Latorraca MQ (2003). Enhanced immunological response influenced by probiotics during the recovery of experimental malnutrition. Revista Brasileira de Nutrição Clínica 18: 157-162. [ Links ]
Fuller RA (1991). Probiotics in human medicine. Gut 32: 439-442. [ Links ]
Fuller RA (1989). A review: probiotics in man and animals. Journal of Applied Bacteriology 66: 365-378. [ Links ]
Garcia-Urkia N, Arsenio AB, Zubillaga Azpiroz I (2002). Beneficial effects of Bifidobacterium lactis in the prevention of bacterial translocation in experimental short bowel syndrome. Cirurgia Pediatrica 15: 162-165. [ Links ]
Hulsewe KWE, van Acker BAC, von Meyenfeldt MF, Soeters PB (1999). Nutritional depletion and dietary manipulation: effects on the immune response. World Journal of Surgery 23: 536-544. [ Links ]
Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, Sakata T (1999). Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Digestive Diseases & Sciences 44: 2119-2123. [ Links ]
Isolauri E (2001). Probiotics in human disease. American Journal of Clinical Nutrition 73(Suppl): 1142-1146. [ Links ]
Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T (1991). A human Lactobacillus strain (Lactobacillus GG) promotes recovery from acute diarrhea in children. Pediatrics 88: 90-97. [ Links ]
Isolauri E, Sutas Y, Kankaapaa P, Arvilommi H, Salminen S (2001). Probiotics: effect on immunity. American Journal of Clinical Nutrition 73(Suppl): 444S-450S. [ Links ]
Link-Amster H, Rochart F, Soudan KY, Mignot O, Aeschlimann JM (1994). Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunology and Medical Microbiology 10: 55-64. [ Links ]
Lu L, Walker A (2001). Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. American Journal of Clinical Nutrition 73(Suppl): 1124S-1130S. [ Links ]
Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C (2001). Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580-591. [ Links ]
McGhee J, Mestscky M, Dertzkaugh J, Eldridge M, Hilrasawa M, Kilyono H (1992). The mucosal immune system from fundamental concepts to vaccine development. Vaccine 10: 75-88. [ Links ]
McNaught CE, MacFie J (2001). Probiotics in clinical practice: a critical review of the evidence. Nutrition Research 21: 343-353. [ Links ]
Meredith JW, Ditesheim JA, Zaloga G (1990). Visceral protein levels in trauma patients are greater with peptide diet than with intact protein diet. Journal of Trauma 30: 825-828. [ Links ]
Perdigon G, Alvarez S (1992). Probiotic and the immune stage. In: Probiotic. R. Fuller, Ed., Chapman and Hall Sci. Ltd., London, pp 145. [ Links ]
Perdigon G, Alvarez S, Ruiz Holgado AP (1991). Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on secretory immune response and protective capacity in intestinal infections. Journal of Dairy Research 58: 485-496. [ Links ]
Perdigon G, Alvarez S, Rachid M, Aguero G, Gobbato N (1995). Immune system stimulation by probiotcs. Journal of Dairy Science 78:1597-1606. [ Links ]
Perdigon G, Macias MEN, Alvarez S, Oliver G, Ruiz Holgado AP (1990). Prevention of gastrointestinal infection using immunobiological methods with fermented with Lactobacillus casei and lactobacillus acidophilus. Journal of Dairy Research 57: 255-264. [ Links ]
Poxton I, Brown A, Sawyer A, Fergunson A (1997). The mucosal anaerobic gram negative bacteria of the colon. Clinical Infectious Diseases 25: 111-113. [ Links ]
Puri P, Mahapatra SC, Bijlani RL, Prasad HK, Nath I (1994). Feed efficiency and splenic lymphocyte proliferation response in yogurt and milk-fed mice. International Journal of Food Sciences and Nutrition 45: 231-235. [ Links ]
Reynolds JV, OFarrelly C, Feighery C, Murchan P, Leonard N, Fulton G, OMorain C, Keane FB, Tanner WA (1996). Impaired gut barrier function in protein malnourished patients. British Journal of Surgery 83: 1288-1291. [ Links ]
Reynolds JV, Redmond H, Ueno N, Steigman C, Ziegler MM, Daly JM, Johnston Jr RB (1992). Impairment of macrophage activation and granuloma formation by protein deprivation in mice. Cellular Immunology 139: 493-504. [ Links ]
Roediger WEW (1980). Role of anaerobic bacteria in the metabolic welfare of colonic mucosa in man. Gut 21: 793-798. [ Links ]
Sakata T, Kojima T, Fujieda M, Miyakozawa M, Takahashi M, Ushida K (1999). Probiotic preparation dose-dependently increase net production rates of organic acids and decrease that of ammonia by pig cecal bacteria in batch culture. Digestive Diseases & Sciences 44: 1485-1493. [ Links ]
Silk DBA (1987). Toward the optimization of enteral nutrition. Clinical Nutrition 6: 61-74. [ Links ]
Tannock GW, Savage D (1974). Influences of dietary and environment stress on microbiological population in the gastrointestinal tract. Infection and Immunity 9: 591-598. [ Links ]
Thoreux K, Balas D, Bouley C, Senegas-Balas F (1998). Diet supplemented with yogurt or milk fermented by Lactobacillus casei DN-114 001 stimulates growth and brush-border enzyme activities in mouse small intestine. Digestion 59: 349-359. [ Links ]
Tuomola E, Ouwehand A, Salminen S (1999). The effect of probiotic bacteria on the addesion of pathogens to human intestinal mucus. FEMS Immunology and Medical Microbiology 26: 137-142. [ Links ]
Windsor JA, Hill GL (1988). Protein depletion and surgical risk. Australian and New Zealand Journal of Surgery 58: 711-715. [ Links ]
Zaloga GP, Ward KA, Prielipp RC (1991). Effect of enteral diets on whole body and gut growth in unstresses rats. Journal of Parenteral and Enteral Nutrition 15: 42-47. [ Links ]
Received on October 24, 2003.
Accepted on February 11, 2004.














