Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.28 n.2 Mendoza abr./ago. 2004
Intrahost distribution and trasmission of a new species of cyclopoid copepod endosymbiotic to a freshwater snail, Pomacea canaliculata (Caenogastropoda, Ampullariidae) from Argentina
C. D. Gamarra-Luques, I. A. Vega, E. Koch and A. Castro-Vazquez
Laboratory of Physiology (IHEM-CONICET), Department of Morphology and Physiology, School of Medicine, University of Cuyo, Casilla de Correo 33, M5500 Mendoza, Argentina.
Address correspondence to: Dr. Alfredo Castro-Vazquez. Laboratorio de Fisiologia, Facultad de Ciencias Médicas, UNCuyo. Casilla de Correo 33. (5500) Mendoza, ARGENTINA. TEL: +54 (261) 413-5006 int. 2715.FAX: +54 (261) 449-4117 E-mail: acv@fcm.uncu.edu.ar
Abstract: A new species of cyclopoid copepod, Ozmana huarpium, is described as a symbiont to Pomacea canaliculata (Lamarck 1822) (Caenogastropoda, Ampullariidae). Rather large numbers (about one hundred copepods per snail) were found, although there was no evidence of harm to the host. To our knowledge, O. haemophila (symbiont to P. maculata), and the currently described species, O. huarpium, are the only copepod species ever recorded as endosymbionts to freshwater invertebrates. While O. haemophila is restricted to the haemocoel of its host, O. huarpium predominate in the penis sheath, the ctenidium and the mantle cavity, figuring in these pallial organs 63-65 % of total mature forms. The sex ratio of the symbiont is skewed to the female side in these organs, specially in male hosts. The hypothesis that a special female tropism for the male hosts pallial organs might ensure interindividual transmission of the symbiont was tested, with indications that the symbiont is mainly transmitted during copulation.
Key words: Ozmanidae. Ozmana haemophila. Ozmana huarpium. n. sp.. Pallial organs. Haemocoel.
Introduction
Pomacea maculata Perry 1810 (Caenogastropoda, Ampullariidae) hosts a cyclopoid copepod, Ozmana haemophila Ho & Thatcher 1989, Ozmanidae (Ho and Thatcher, 1989) that occurs in rather small numbers (1-14) in the haemolymph of this large snail (up to 14 cm in shell length). The life cycle of O. haemophila is unknown, except for the occurrence of most developmental stages in the snails haemolymph.
In the present paper we describe another ozmanid species, which was found living in high numbers (up to 182) in Pomacea canaliculata (Lamarck 1822) (Caenogastropoda, Ampullariidae). This snail is smaller than P. maculata (only up to 6 cm in shell length) and it is distributed in both tropical and subtropical zones, including the Plata and Amazon basins (Estebenet and Martín, 2002); in parts of the latter basin P. canaliculata may be sympatric to P. maculata. Various other organisms have been either reported or proposed as symbiotic partners or parasites to P. canaliculata: a prokariont (Castro-Vazquez et al., 2002), two ciliates (Parasicuophora ampullariarum and P. corderoi; Gascón, 1975), a rotifer (genus Abrochtha Bryce 1910, Philodinavidae, Gamarra-Luques & Castro-Vazquez, unpublished findings), and some temnocephalid platyhelmynthes (Damborenea, 1998; Damborenea and Cannon, 2001), hirudinea (Damborenea and Gullo, 1996) and digenea (Hamann, 1992; Keawjam et al., 1993).
Material and Methods
Copepods were obtained mostly from mature snails of a cultured strain of Pomacea canaliculata, although some observations were also made on field-collected animals (Rosedal and Regatas Lakes, Palermo Park, Buenos Aires, Argentina). Since then, the lake was cleaned up and the original population was extinguished, but voucher (alcohol preserved) specimens of the original population and of the cultured strain were deposited at the collection of Museo Argentino de Ciencias Naturales (Buenos Aires, Argentina; lots MACN-In 35707 and MACN-In 36046, respectively). Histological observations were made on material fixed in Bouins fluid, embedded in paraffin and stained with haematoxylin and eosin.
Cultured snails were maintained under conditions of artificial lighting (14 h per day) and temperature (23-25 °C), and were fed ad libitum on a mixed diet (lettuce, rodent food pellets and toilet paper).
Copepods were looked for in the heart and the perintestinal and peripharyngeal haemocoel sinuses under a stereoscopic microscope. The mantle cavity (and dependent organs: ctenidium, osphradium, vagina and penis sheath) and the intestine (both mucosa and content), were also investigated for copepods (a thin water jet was produced with a hypodermic syringe to wash out the copepods from the different surfaces and collect them in Petri dishes).
After fixation in 70% alcohol for 30 min, the copepods were quickly passed through 80% alcohol, and stained (22 mg/dL eosin, 30 mg/dL phosphotungstic acid, dissolved in 96% alcohol). They were then washed in 96º alcohol, cleared in phenol/xylene (1:3, v/v) and mounted in Eukitt.
One hundred and thirty two female individuals and 98 male individuals were examined for morphological description of the new species. All these specimens were obtained from the haemocoel, mantle cavity, ctenidium and penis sheath´s groove of living snails. Also, 62 immature individuals of the same origin (all of them in copepodid stages) were studied. Type material was deposited at the Invertebrate collection of the Museo Argentino de Ciencias Naturales (MACN-In), Buenos Aires, Argentina, and at the Museo de La Plata (MLP), La Plata, Argentina.
For studying the intrahost distribution of copepods, adult female (shell length 36.7-50.2 mm) and male snails (shell length 39.5-51.6 mm) were sacrificed, and copepods carefully recovered in Petri dishes (as described above), counted and classified as either immatures, adult males or adult females; the number of egg-bearing females was also recorded. The sex ratio was calculated as M/(M+F), M and F being number of males and females, respectively.
For studying the way of transmission of the symbiont two different sets of observations were run. First, non-infested adult snails were used (shell length >35 mm), which were obtained by culturing newly hatched snails of both sexes in the laboratory, preventing their contact with infested snails. Infested snails were obtained from aquaria in which at least one infested snail was found (we have previously determined that infestation of snails by the copepod was always present in snails inhabiting with infested snails). Different experimental groups of adult snails were made: (1) infested males with non-infested females; (2) infested females with non-infested males; (3) infested males with non-infested males; (4) infested females with non-infested females. The initially non-infested animals were sacrificed 15- 20 and 30-40 days after the groups were formed, and the total number of copepods (mostly mature males and females) in the perintestinal sinus and the pallial organs was recorded.
Furthermore, we observed the behaviour of adult copepods of both sexes that were kept in Petri dishes containing paper-filtered aquarium water at 24 ºC. Water was changed daily and the copepods survival was recorded daily for 10 days (a copepod was considered dead when no movements were elicited by gentle stimulation with a micropipette tip). The fate of eggs was also recorded during the same period.
Statistical evaluation of differences between two groups was made by means of Students t test, while multigroup comparisons were made by Kruskal-Wallis one-way analysis of variance, followed by Dunn test.
Results
Family Ozmanidae Ho et Thatcher 1989.
Genus Ozmana Ho et Thatcher 1989.
Ozmana huarpium Gamarra-Luques et Castro-Vazquez, n. sp.
Description:
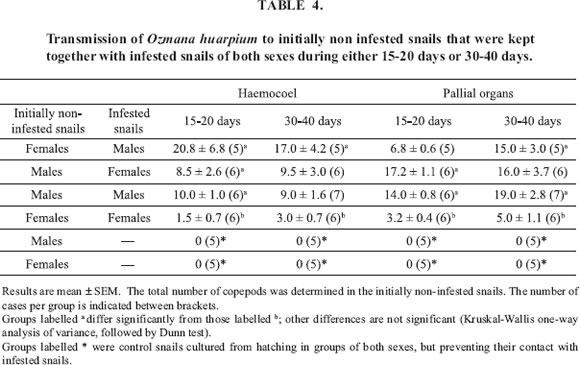
Female (Figs.1A, 2A-B, 3D):
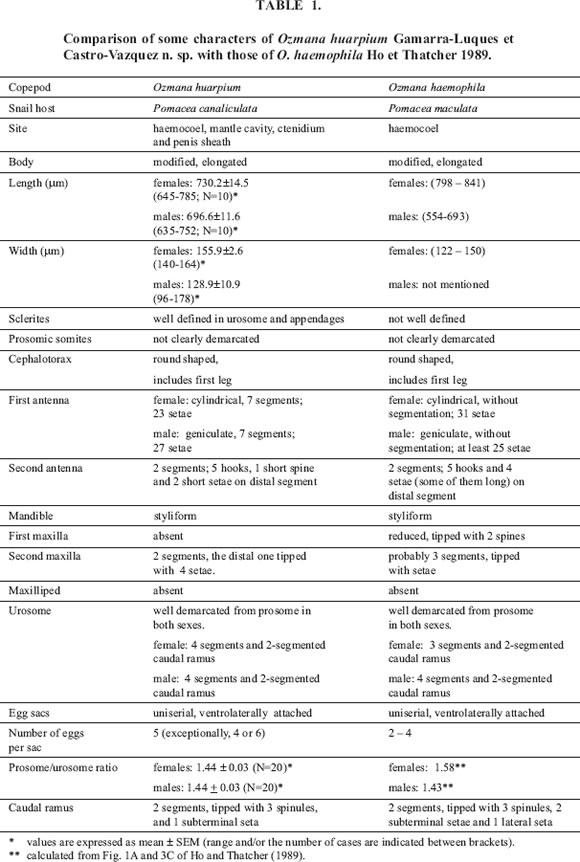
Body modified, elongated. Urosome distinctly narrower than prosome. Urosome and appendages with well defined sclerites. Cephalothorax rounded, including first leg-bearing somite. First antenna cylindrical, seven-seg-mented, worn with numerous setae. Second antenna with prehensile hooks, labrum in the midline, partly covered by a proboscis. Urosome formed by four segments of approximately equal length, with anal somite distinct from caudal ramus. Ovipores on the ventrolateral surface of the first urosomic somite; mouth-like ventromedial structure on the same somite. Egg sacs attached to the ovipores, with eggs in linear disposition, each sac containing 4-6 eggs (mostly 5). Four pairs of biramous prosomic legs, with a well developed armature of spines and setae; a fifth pair is uniramous (further details in Tables 1 and 2).
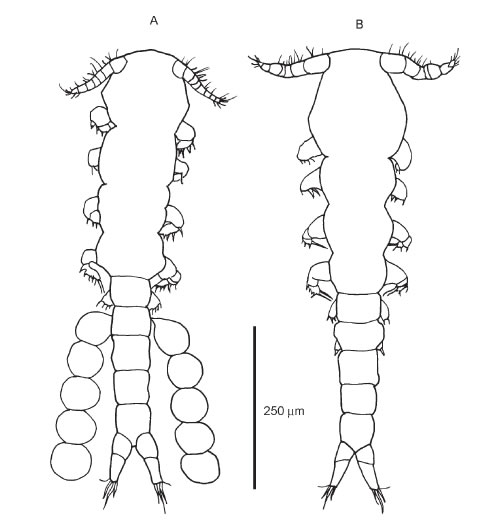
FIGURE 1. Dorsal views of an adult female (A) and a male (B).
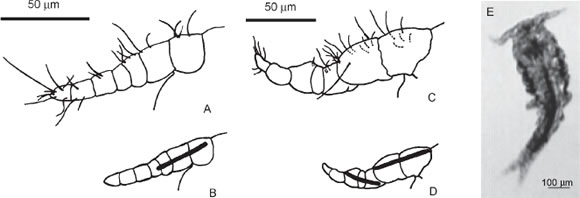
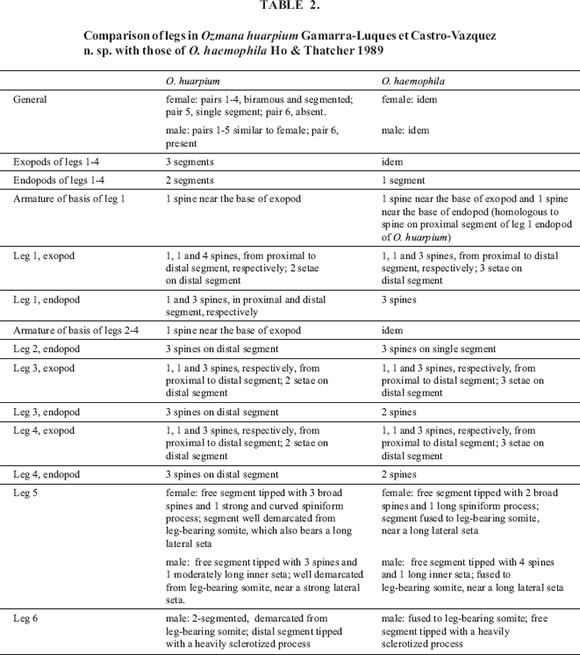
FIGURE 2. Segments and setae of the female (A) and male (C) first antenna. Disposition of muscles in the female (B) and male (D) first antenna. A living male specimen while flexing the left first antenna (E).
FIGURE 3. Ventral view of the cephalothorax and the first thoracic segment of a male (A). Detail of the second antenna (B). In (C) a lateral view of the proboscis (p), labrum (la), mandible (md) and second maxilla (mx) is depicted. Ventral view of the lower prosome and upper urosome of a female (D) and a male (E).
Male (Figs. 1B, 2C-D, 3A-C, 3E):
Body roughly as in female. First antenna geniculate, seven-segmented, mobile, worn with numerous setae. Urosome formed by four segments of approximately equal length. First urosomic somite with a ventromedial ridge corresponding to a mouth-like structure in the female. Four pairs of biramous prosomic legs, with a well developed armature of spines and setae; a fifth pair is uniramous. A sixth, spiniform pair on the genital somite (further details in Tables 1 and 2).
Host:
Pomacea canaliculata (Lamarck 1822) (Caenogastropoda, Ampullariidae)
Types:
Holotype (female), MACN-In 36047; allotype (male), MACN-In 36048.
Paratypes:
Females with eggs (MACN-In 36049 and MLP 25807, 10 individuals each), females without eggs (MACN-In 36050 and MLP 25808, 10 individuals each), and males (MACN-In 36051 and MLP 25806, 10 individuals each).
Location:
All these specimens were obtained from the haemocoel, mantle cavity, ctenidium and penis sheath´s groove of Pomacea canaliculata cultured in Mendoza, Argentina.
Type locality:
The culture strain was originated from animals collected at the Rosedal Lake (Palermo Park, Buenos Aires,Argentina), and since we also confirmed the presence of these copepods in animals collected at the Regatas Lake (within the same park), we are hereby designating Palermo, in Buenos Aires city, as the type locality.
Etymology:
huarpium (= of the Huarpes), a genitive noun, refers to the Huarpe people, whose culture once predominated in Mendoza, Argentina.
Survival and behaviour of copepods outside the snails
Eighty adult copepods of both sexes were still alive one day after being removed from the snails and placed in aquarium water. All the egg-bearing females had released their eggs by that day, and neither hatching of the released eggs was observed during the 10 days period of observation, nor additional egg sacs appeared attached to the females ovipores. Copepods died during the subsequent days, a few of them having survived up to seven days in such conditions.
Both the adults and the copepodid forms of O. huarpium were unable to swim in aquarium water; they were only able to crawl over short distances on the bottom surface of the Petri dish and over the mucous clumps, to which they usually remained attached and motionless. When stimulated with a micropipette they showed a clear thigmotropic response, turning to the pipette tip and attaching to it. When obliged to leave they seldom attempt to flight by crawling, but they started a series of ventral flexions and extensions of the urosome over the prosome, from which no locomotion resulted.
Spontaneous behaviour of both females and males also included rapid and simultaneous abduction and adduction movements of the right and left first antennas. The males are also able to rapidly flex and extend their geniculate first antennas (Fig. 2E) independently from each other.
Two striated muscles, a proximal and a distal one, could be recognized in the first antenna of fixed, eosinestained males (Fig. 2D); the distal muscle appeared responsible for flexion and extension of this antenna, and it seemed not developed in females (Fig. 2B). The proximal muscle extended in both sexes from the joint of the head and the first segment; it extended to the distal part of the second antennal segment in males, while in females it appeared longer, extending up to the limit between the third and fourth antennal segments.
Prevalence, mean intensity and intrahost distribution of copepods according to hosts sex
As expected from previous, non-systematic observations, prevalence was 100% in both sexes. Mean intensities in male than female snails were 105.9 ± 15.4 (N=10) and 66.5 ± 9.7 (N=10) mature copepods per snail, respectively (P<0.05, Students t test). The ranges were rather similar, however: 36-182 copepods per male snail and 37-163 per female snail.
The number of adult copepods in the haemocoel and pallial organs of female hosts did not differ significantly (Kruskal-Wallis test followed by Dunn test). In male hosts, the mean number of adult copepods was higher in the pallial organs than in the haemocoel (Table 3) although the difference did not reach significance. However, the number present in the pallial organs was significantly higher in male than in female hosts (same tests). Copepods occurred mainly on the ctenidium of both sexes and in the penis sheath´s groove of males, but they were never found on the osphradium.
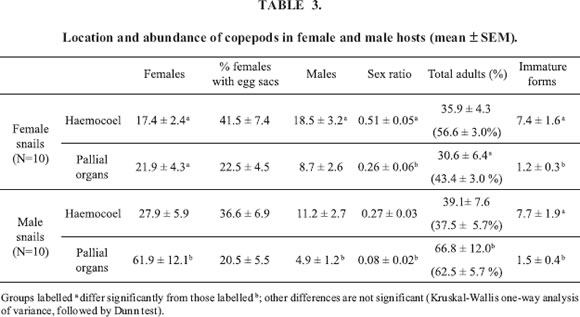
The sex ratio varied substantially according to host´s sex and organs (from 0.51 in the haemocoel of female snails to 0.08 in pallial organs of male snails). In both sexes, immature copepodid forms were rather frequent in the haemocoel, but not in the pallial organs (Table 3).
It should be noted that when copepods were observed in histological sections of the snail (penis sheath, seminal vesicle) no tissue reaction was seen surrounding the symbiont in any case.
Transmission of the symbiont
The larger number of adult copepods found in male snails, as well as the lowest sex ratio found in their pallial organs (including the penis sheath), suggested that the fertile females crowding in the copulatory organ of the snail may be playing a role in the transmission of the symbiont. Therefore, an experiment to test the possibility of a sexual transmission of O. huarpium was made.
As expected, individuals of O. huarpium were readily transmitted to formerly non-infested snails by keeping them together with infested snails for 15-20 days (Table 4). A relatively high number of copepods was found in formerly non-infested hosts after cohabitation with infested snails of the other sex, and also, a similarly high number of copepods was found in formerly non-infested male snails that were grouped with infested males. A significantly smaller number of copepods was observed when initially non-infested females were put together with infested snails of the same sex (Kruskal-Wallis and Dunns test). The expected skewed distribution in favour of the pallial organs of males was not found. Also, after a longer cohabitation (30-40 days), the copepod abundance was rather similar to that foundafter 15-20 days only.
As expected, no copepods were found in groups of both female and male non-infested snails that were prevented from contact with infested snails.
Discussion
Ozmana huarpium n. sp. and O. haemophila may be clearly differentiated according to morphological characters, and it appears reasonable that the major cladogenic event proposed by Ho (1994), implying the association of a cyclopoid copepod to a freshwater ampullariid snail, would have later resulted in more than one ozmanid species that could have co-evolved with species of the genus Pomacea.
It is notable that the number of copepods harboured by a single snail is much higher in Pomacea canaliculata than in P. maculata, and these large numbers have allowed us to study their behaviour, as well as their intrahost distribution and transmission. It is then possible that O. haemophila might also be found in the pallial organs of P. maculata, if specially designed observations were conducted.
Female O. huarpium are usually more abundant than males, and they show a preferential distribution in the pallial organs of male snails, which showed the lowest sex ratio (0.08) and which includes the penis sheath that is inserted into the female´s mantle cavity during copulation. Although adult O. huarpium may be sometimes found in unusual places (e.g., seminal vesicle, heart ventricle or peripharyngeal sinus) they are mostly found in the perintestinal sinus, the penis sheath, ctenidium and mantle cavity. Immature forms are more abundant in the haemocoel than in the pallial organs. Other organs in which copepods were carefully looked for with no success, were: (1) vagina, (2) intestinal mucosa and gut content, and (3) osphradium.
O. huarpium is readily transmitted from male to female snails, and from females to males, as a consequence of cohabitation in the same aquaria (Table 4). Transmission was likely to occur during copulation, which provides a prolonged contact (10-18 hours, Albrecht et al., 1996) of the penis sheath with the female´s mantle cavity; also, one end of the heavily populated ctenidium remains in close proximity to the partners mantle skirt during copulation.
Since males of P. canaliculata are known to copulate with other males, particularly when no females are present (Albrecht et al., 1996), the transmission in maleonly aquaria would also be explained by penis sheath penetration into the mantle cavity. Since no female-female "copulatory" interactions are known, the smaller degree of infestation observed after cohabitation of initially non-infested females with infested females (Table 4) also favours the idea that O. huarpium is maintained in the populations of P. canaliculata by copulation. Transmission in female-only aquaria could occur mainly during brief contacts of the mantle borders of females during group interactions.
We also propose that the male host is crucial for transmission of the copepod in the snail populations, since female copepods predominate in the pallial organs of male snails and since infested male P. canaliculata bear a higher number of symbionts than female snails.
Infestation by copepods passing from one snail to someone other in the surroundings is unlikely, since both mature individuals of O. huarpium and their copepodid larvae seem to be unable to swim, and their crawling displacements seem short ranging. In Ismaila occulata Ho 1987, a poecilostomatoid copepod inhabiting the haemocoel of marine gastropods (Ho, 1987), the infesting stage is the first copepodid larva and Ho and Thatcher (1989) have proposed that this may also be true for O. haemophila, whose habitat appears restricted to the haemocoel of the freshwater snail P. maculata. In view of the findings of Tables 3 and 4, however, we believe that the main infesting stage for O. huarpium should be the adult female, since females may easily crawl from the penis sheath groove into the mantle cavity of the female snail, and vice versa. Also, the immature forms, as well as the adult males, are comparatively infrequent in the pallial organs. We hypothesize that the armature of O. huarpium may permit to readily traverse the soft tissues of its host, specially the rather thin layer of tissues separating the mantle cavity from the perintestinal sinus.
The predominance of female copepods in the pallial organs is interesting, since it is possible that the mantle cavity and its organs should exert some form of attraction for adult females, or, alternatively, that the haemocoel should be exerting it for males. Another interesting possibility would be that some unknown condition in the mantle organs might be shifting somehow sexual differentiation to the female side, while the haemocoel might be shifting it to the male side.
The rather large number of adult copepods found in the perintestinal sinus, the more equilibrated sex ratio, as well as the higher incidence of immature forms, all these data suggest that reproduction of O. huarpium occurs mainly in the haemocoel of its host. From there, many females (and some males) may traverse the rather thin layer of tissues separating the perintestinal sinus from the mantle cavity. Once there, the interlamellar spaces of the ctenidium and the penis sheath´s groove may provide places for fixation and shelter against the water currents in the mantle cavity (in this context, it is puzzling that the osphradium, also a multilamellar structure, was always found devoid of copepods). Finally, the large penis sheath and the lengthy copulation of P. canaliculata may be providing the occasion for some female copepods (and some males) to crawl into the female´s mantle cavity and ctenidium during male-tofemale transmission. Alternatively, during female-tomale transmission, they may crawl from the female´s ctenidium to the nearby attached penis sheath, and from there to the rest of the male´s mantle cavity. Afterwards, some degree of reproduction may occur in the recently colonized mantle organs (since a small proportion of immature forms are also found there) and finally, many males (and some females) may cross the barrier separating the mantle cavity from the perintestinal sinus, thereby closing the circle.
Aknowledgements
This work was supported by grants from CONICET, ANPCyT and the National University of Cuyo (Argentina). We are grateful to Drs. Cristina Damborenea and Néstor J. Cazzaniga for critical reading of the manuscript.
References
Albrecht EA, Carreño NB, Castro-Vazquez A (1996). A quantitative study of copulation and spawning in the South American apple-snail,Pomacea canaliculata (Prosobranchia, Ampullariidae). Veliger 39: 142-147. [ Links ]
Castro-Vazquez A, Albrecht EA, Vega IA, Koch E, Gamarra-Luques C (2002). Pigmented corpuscles in the midgut gland of Pomaceacanaliculata and other Neotropical apple-snails (Prosobranchia, Ampullariidae): A possible symbiotic association. Biocell 26: 101-109. [ Links ]
Damborenea MC, Gullo BS (1996). Hirudíneos asociados a la cavidad paleal de Pomacea canaliculata (Lamarck 1822) (Gastropoda Ampullariidae) en el Balneario Bagliardi, Río de la Plata, Argentina. Neotropica 42: 97-101. [ Links ]
Damborenea MC (1998). Distribution patterns of temnocephalids commensal with Crustacea and Mollusca from Argentina. Hydrobiologia 383: 269-274. [ Links ]
Damborenea MC, Cannon LRG (2001). On Neotropical Temnocephala (Platyhelminthes). J Nat Hist 35: 1103-1118. [ Links ]
Estebenet AL, Martín PR (2002). Pomacea canaliculata (Gastropoda: Ampullariidae): Life-history traits and their plasticity. Biocell 26: 83-89. [ Links ]
Gascón A (1975). Dos ciliados del género Parasicuophora parásitos de Pomacea canaliculata. Revista de Biología del Uruguay 3: 111-125. [ Links ]
Hamann MI (1992). Catadiscus pomaceae sp. n. (Trematoda, Paramphistomatidae) from Pomacea canaliculata (Lamarck 1801)(Prosobranchia, Ampullariidae). Mem I Oswaldo Cruz 87: 9-14. [ Links ]
Ho JS (1987). Larval stages of Ismalia occulata Ho, 1981 and the affinity of Splanchnotrophidae (Copepoda: Poecilostomatoida).Researches on Crustacea 16: 67-83. [ Links ]
Ho JS (1994). Origin and Evolution of the Parasitic Cyclopoid Copepods. Int J Parasitol 24: 1293-1300. [ Links ]
Ho JS, Thatcher VE (1989). A new family of cyclopoid copepods (Ozmanidae) parasitic in the hemocoel of a snail from the Brazilian Amazon. J Nat Hist 23: 903-911. [ Links ]
Keawjam RS, Poonswad P, Upatham ES, Banpavichit S (1993). Natural parasitic infection of the golden apple snail, Pomacea canaliculata. Southeast Asian J Trop Med Public Health 24: 170-177. [ Links ]
Received on March 5, 2004.
Accepted on June 9, 2004.