Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.28 n.3 Mendoza ago./dez. 2004
Tissue expression of platelet endothelial cell adhesion molecule-1 at pre and postnatal murine development
Graciela Cristina Calabrese* and Rosa Wainstok**.
* Cátedra de Biología Celular e Histología, Departamento de Ciencias Biológicas. Facultad de Farmacia y Bioquímica. Universidad de Buenos Aires. Junin 954/6, 1113 Buenos Aires, Argentina.
** Departamento de Química Biológica. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Ciudad Universitaria, Pabellón II, 4º Piso, Nuñez, 1428 Buenos Aires, Argentina.
Address correspondence to: Dra. Rosa Wainstok. Departamento de Química Biológica. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Ciudad Universitaria, Pabellón II, 4º Piso, Nuñez, 1428 Buenos Aires, ARGENTINA. FAX: (+54-11) 4576-3342.
E-mail: rwains@qb.fcen.uba.ar
ABSTRACT: Endothelial cells, at the cell-cell borders, express PECAM-1, and have been implicated in vascular functions. The monoclonal antibody MEC 13.3 recognizes PECAM-1 molecule from mouse vessels and allows to analyze the ontogeny of mouse endothelium. At the present, little is known about the molecular basis of differentiation pathways of endothelial cells, that enables its morphological heterogeneity. The purpose of this study was to analyze the pattern of PECAM-1 expression, employing monoclonal antibody MEC 13.3, in cellular suspensions obtained from different mouse organs at pre and postnatal stages.
Fluorescence activated cell sorter analysis showed a different profile of the glycoprotein expression in a cell population with size and granularity selected by 1G11 endothelial cell line. The expression differs from prenatal to postnatal developmental stages in a given organ, and among the organs studied.
Another cell population, with a size and granularity higher than 1G11 endothelial cell line, coexists in cellular suspensions obtained from liver, gut and brain. These cells could be related to those detected by means of immunoenzyme methods which showed a non-differentiated morphology.
The different PECAM-1 pattern expression could reflect potential organ-specific differentiation pathways during development and according to organs environment.
The existence of another cell population with a size and granularity higher than 1G11 endothelial cell line required a phenotypic characterization.
Key words: Endothelial cells, PECAM-1, vasculogenesis, mouse organs.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1), an integral membrane glycoprotein, is highly expressed at endothelial cells (ECs), and at moderate levels on the surface of platelets and leucocytes (Newman, 1997). It was originally assigned to the immunoglobulin–like cell adhesion molecules family, however, nowadays it appears to fulfill the criteria established for inclusion in the immunoreceptor tyrosine-based inhibitory motifs (Newman, 1999). PECAM-1 is composed by six extracellular domains, a short transmembrane region, and a cytoplasmatic tail of variable length. It has been involved in a number of vascular functions including initial formation of ECs contacts, vascular permeability, angiogenesis, cell migration, leukocyte-endothelial adhesion and transendothelial migration (Springer, 1994; Piedboeuf et al. , 1998).
PECAM-1 is localized at the cell-cell borders of adjacent ECs, its role as an adhesion molecule is well known, and, recently, its functions in cell signaling have also been demonstrated (Sun et al., 2000). PECAM-1/ PECAM-1 homophilic interactions are responsible for concentrating this molecule at ECs intracellular junctions. In addition, several studies have suggested that PECAM-1 may also be capable of interacting heterophilically with other components of the cell surface in a process that is dependent upon the presence of calcium and glycosaminoglycans (De Lisser et al., 1994; Yan et al., 1995).
Different isoforms of PECAM-1, in which one or more clusters of polypeptides were missing from the cytoplasmic domain, have a role in EC-EC interactions and morphogenesis (Baldwin et al., 1994; Sheibani et al., 2000).
The monoclonal antibody (mAb) MEC 13.3 recognizes PECAM-1 molecule present in the membrane of ECs from mouse vessels. Its high specificity for ECs allows the use of this mAb to analyze the ontogeny of mouse endothelium (Vecchi et al., 1994). We have previously described single cells with a typical non-differentiated morphology detected by mAb MEC 13.3 in mouse embryo development (Calabrese et al., 1998).
According to their morphology, physiology, cell biology and biochemistry microvascular endothelium of normal adult organism has been divided into different phenotypes: continuous, fenestrated and sinusoid. These different characteristics reflect potential differentiation pathways of ECs in a given tissue or organ, attributed to soluble factors or cell-cell interactions. At the present time, little is known about the molecular basis of ECs diversity.
The purpose of this study was to analyze, “in vivo”, the cell type detected by mAb MEC 13.3 in cellular suspensions obtained from pre and postnatal murine organs, in order to establish a possible relationship between PECAM-1 expression and microvascular endothelium diversity.
The results support the existence of two different cell populations that co-exist during the organ development, one of them with size and granularity characteristics of 1G11 ECs and the other one with a higher size and granularity.
Materials and Methods
Animals and organs
Balb-c mouse embryos at 14.5 and 17.5 days postcoitum were removed by Cesarean sections and dissected from the decidual mass. Postnatal organs were obtained from 10-day-old animals.
Organ cellular suspensions
Once the embryo was dissected from its surrounding membranes, the gut, liver and brain were removed (Loo and Cotman, 1998). The same organs from postnatal animals were also taken off.
Organ cellular suspensions were obtained as described by Lemberg (Lemberg et al., 1998). Each organ was kept in a pre-buffer containing EDTA solution at 37ºC for 20 min before collagenase type IV disruption was done (Sigma Chemical, St. Louis, USA) by means of an enzymatic solution of 0.05% wt/vol. for 15 min. at 37ºC. Enzymatic activity was stopped on ice bath. All suspensions were filtered through a nylon-wool mesh and washed twice before using. The percentages of viable cells were determined by staining cell population with trypan blue exclusion (0.2% saline). A hemocytometer counting technique method demonstrated approximately 95% viable cells in every experiment. Organ cellular suspensions (1x106 cells/ml) were mounted on polilysine treated slides, fixed 10 minutes in cold acetone and then rehydrated in phosphate buffer saline (PBS), pH 7.4, followed by immunostaining, or washed in DMEN with 10% of fetal calf serum for fluorescence activated cell sorter (FACS) analysis.
Antibodies
The following monoclonal antibodies obtained by courtesy of Dr. Annunciata Vecchi (Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy) were used for immunostaining: MK 1.9, CRL 1878 and MEC 12; anti-ICAM, anti-VCAM and anti-Sendo antigen, respectively. Rat anti-murin PECAM-1 antibody MEC 13.3, was employed both for immunostaining and flow cytometric analysis.
Background staining in FACs analysis was evaluated by means of isotype control FITC-conjugated rat IgG2a monoclonal immunoglobulin (BD PharMingen, San Diego, CA).
Fluorescence activated cell sorter analysis
The studies for ECs were performed in parallel with studies of 1G11 lung endothelial cells. Samples were run on a FACScan flow cytometer (Cytoron Absolute, Ortho Diagnostic Systems, Johnson-Johnson Company) and data were collected from 10.000 events. An isotype-matched antibody was used as negative control. Cell analysis was carried out considering the gate of the population selected by forward angle (FW-SC) and 90º light scatter (RT-SC) from an ECs culture obtained from murine lungs (Dong et al. 1997).
Organ cellular suspensions were washed in DMEM with 10% fetal calf serum and counted. Cells (1x106 /sample) were incubated on ice with 100 ml of mAb MEC 13.3 for 30 min, washed with PBS, and incubated with 100 ml of a fluoresceinated monoclonal goat anti-rat IgG, whole molecule (Sigma Chemical, St. Louis, USA) diluted 1:20 with PBS, washed twice, resuspended in 2% paraformaldhyde PBS and stored in dark at 4ºC until being analyzed.
Data analysis was performed by using Lysys II software and WinMDI 2.8 software.
Immunoenzyme method
Immunostaining was performed as it was previously described (Calabrese et al., 1998). Cellular suspension was mounted on polilysine-treated slides, fixed in cold acetone at room temperature, and then rehydrated in phosphate buffered saline. After blocking non-specific antibody binding, an overnight exposure to the monoclonal antibody (MK 1.9, CRL 1878, MEC 12 and MEC 13.3) at 4ºC was carried out. Detection of positive cells was performed by a peroxidase-antiperoxidase complex (Accurate Chemical & Scientific Co., USA), counterstained with hematoxylin and mounted with Canadian balsam.
Statistical evaluation
The statistical analysis was performed by using analysis of variance (ANOVA) and Tuckey-Kramer test. Values of P< 0.05 were considered significantly different from controls.
Results
Fluorescence activated cell sorter analysis
Considering that cellular suspensions obtained from different organs were formed by a heterogeneous cell population, it was necessary to perform the studies of ECs in parallel with 1G11 lung ECs, a cellular line established in our laboratory (Dong et al., 1997). Cell analysis was carried out considering the gate selected by forward angle (FW-SC) and 90º light scatter (RT-SC) from the ECs culture obtained from murine lungs processed in the same way as organs suspension samples (Fig. 1).
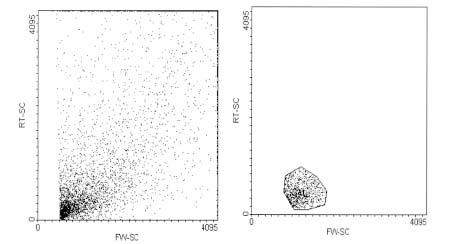
FIGURE 1: Flow cytometric analysis of endothelial cells obtained from murine lungs. (A) Forward (FW-SC) and side scatter (RT-SC) of endothelial cells obtained from murine lungs (1G11). (B) The gated R1 population represents 1G11 endothelial cells.
Flow cytometric analysis of cellular suspension at different development stages In order to study the possible relationship between PECAM-1 expression and the endothelium heterogeneity, FACS analysis of cellular suspensions obtained from different organs at pre and postnatal stages were carried out. Experiments were performed in selected organs with microvascular endothelium morphological differences such as discontinuous ECs: fenestrated capillaries and sinusoids of the gut and liver respectively or continuous ECs of the blood-brain barrier.
Figure 2, column A, shows the PECAM-1 pattern expression of liver samples. Figure 3, column A, shows the pattern expression from gut, of the cells localized in the gate selected by the FW-SC and RT-SC from 1G11 ECs line (R1 gate). Liver and gut suspensions only showed this population at postnatal stage. On the other hand, brain glycoprotein pattern of expression (Fig. 4, column A) had no differences between 17.5 prenatal and postnatal stages.
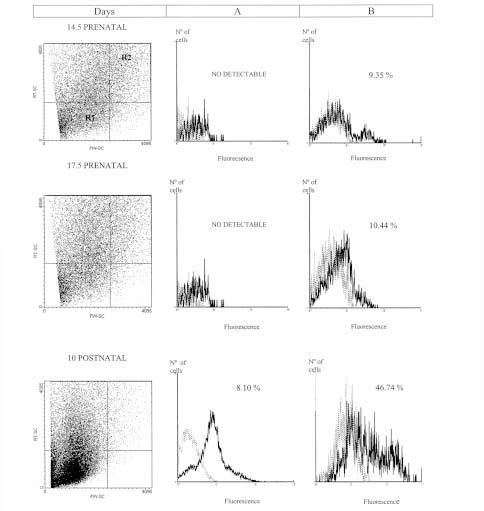
FIGURE 2: Flow cytometric analysis of liver suspensions at different developmental stages. Flow cytometric analysis of cellular suspensions from liver were obtained as described in materials and methods. The PECAM-1 expression selected by 1G11 ECs R1 gate (Column A), or R2 gate (Column B) is showed. Results are representative of three replicated experiments in each case. The percentage of cells is indicated in each quadrant. Dashed line: the isotype control. Solid lines: cells incubated with mAb MEC 13.3.
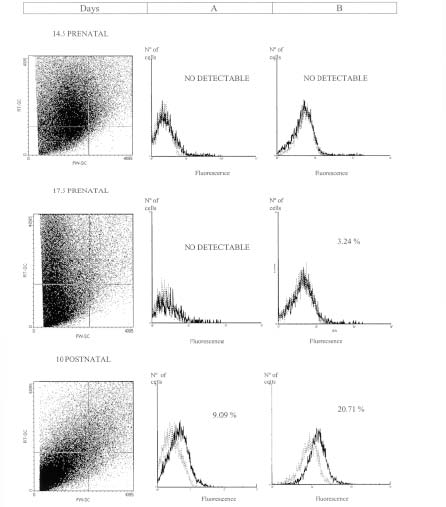
FIGURE 3: Flow cytometric analysis of gut suspensions at different developmental stages. Flow cytometric analysis of cellular suspensions from gut were obtained as it was described in materials and methods. The PECAM-1 expression selected by 1G11 R1 gate (Column A), or R2 gate (Column B) is showed. Results are representative of three replicated experiments in each case. The percentage of cells is indicated in each quadrant. Dashed line: the isotype control. Solid lines: cells incubated with mAb MEC 13.3.
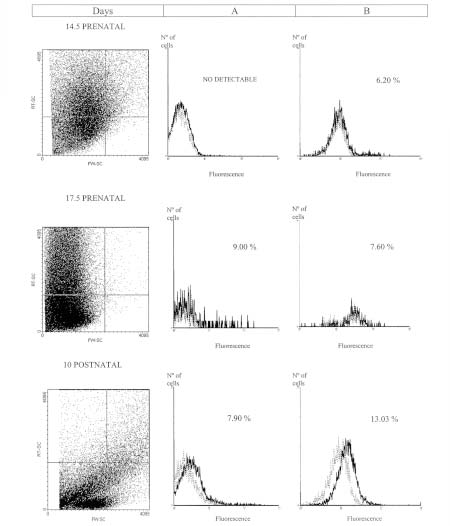
FIGURE 4: Flow cytometric analysis of brain suspensions at different developmental stages. Flow cytometric analysis of cellular suspensions from brain were obtained as it was described in materials and methods. The PECAM-1 expression selected by 1G11 ECs R1 gate (Column A), or R2 gate (Column B) is showed. Results are representative of three replicated experiments in each case. The percentage of cells is indicated in each quadrant. Dashed line: the isotype control. Solid lines: cells incubated with mAb MEC 13.3.
These results suggest that cellular suspensions obtained from different organs at different developmental stages showed a different PECAM-1 pattern expression for the cell population selected by 1G11 ECs R1 gate.
FACS analysis of cellular suspensions from different organs during development showed another cell population, which expressed PECAM-1 with a size and granularity higher than 1G11 cell line. As it can be observed in figure 2, 3 and 4, column B, this cell population was localized in the upper right quadrant (R2 gate). The results obtained from cellular suspensions of liver, gut and brain suggest that two different cell populations, which express the adhesion molecule, co-exist during organ development: one of them with the morphological characteristics of 1G11 ECs, but the other one with a higher size and granularity.
In cellular suspensions obtained from gut and liver there was a significant increase in this cell population throughout embryonic and postnatal stages, perhaps, correlating with the vascular proliferation. For example, in liver suspension (Fig. 2, column B) the percentage of these cells has no differences between prenatal stages. But at postnatal stage, the percentage (46.74%) is significantly higher (p<0.001) than at the embryonic ones. In the gut (Fig. 3, column B) a significantly increase at postnatal stage was observed (p<0.01). Nevertheless, no significant differences could be detected in brain suspensions (Fig. 4, column B), among developmental stages.
These results suggested a particular pattern of PECAM-1 expression for the cells selected by R2 gate, in each organ studied.
Immunohistochemistry studies of cellular suspensions at different development stages
Immunochemistry studies of the cellular suspensions were performed in order to evaluate the morphological characteristics of the ECs isolated.
Cellular suspensions obtained from liver, gut and brain at different developmental stages showed a quiescent endothelium since the immunostaining with MK 1.9, CRL 1878 and MEC 12 (anti-ICAM, anti-VCAM and anti-Sendo antigen, respectively) was negative in the all the cases.
As example, in gut suspension at postnatal stage (10 days), the presence of two types immunoreactive cells with different morphology could be observed (Fig. 5), one of them with a non-differentiated morphology, but with a bigger size and a larger nuclei than the other type.
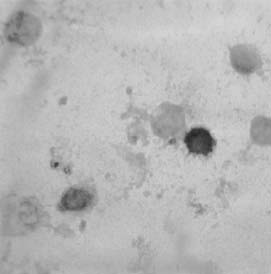
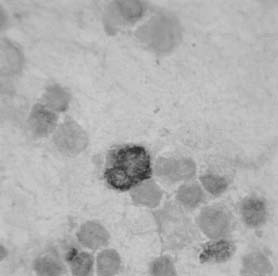
FIGURE 5: PECAM-1 expression as detected by immunoperoxidase staining in gut cellular suspensions at postnatal stage. Specific immunoreactivity was detected in single cells with a different size and morphological characteristics.
Discussion
PECAM-1 is one of the most abundant glycoproteins on the ECs surface. It has been involved in a number of vascular functions including initial formation of EC contacts, vascular permeability and angiogenesis.
PECAM-1 distribution, in adult tissue, has been reported; ECs of different organs expressed similar immunoreactivity detected by mAb MEC 13.3 (Vecchi et al., 1994).
During embryogenesis, angioblasts are known to arise from differentiation of mesodermal cells at early stages. Sprouting of new capillaries from the preexisting network extends this primitive vasculature. A primary capillary plexus is remodeled many times until a mature vascular system is formed (Risau, 1995). Most of the PECAM-1 isoforms are expressed early in the embryo and continue being expressed postnatally, when ECs proliferation is high (Yan et al., 1995; Sheibani et al., 2000).
To determine the tissue expression of PECAM-1 detected by mAb MEC 13.3, we performed FACS analysis, by using different mouse tissues. Tissue selection was carried out on taken in mind the organ distribution of different endothelial phenotypes in normal adult organs.
The results obtained in the present study in cellular suspensions of liver, gut and brain, suggest that two different cell populations, that express the adhesion molecule, co-exist during organ development: one of them with the size and granularity.
We have previously reported the expression of PECAM-1 in the cytoplasm of single cells from mouse at 9.5 days prenatal stage (Calabrese et al., 1998). These single cells exhibit a non-differentiated morphology and their number was significantly increased at day 14.5 when compared to day 9.5, but once the vascular network was established, only low quantities could be detected in all murine embryo frozen sections.
In the present study we described two cell populations that co-exist at early postnatal stages. After the onset of circulation, a primary capillary plexus is remodeled many times until a mature system, consisting of vessels of different diameters and functions, is formed. ECs proliferation, which is increased during embryonic and postnatal development, decreases and is very low in adults. The cell population that expresses PECAM-1 with a size and granularity higher than 1G11 ECs line required a phenotypic characterization to establish a dedifferentiated or mesenchymal phenotype.
All the tissue that we examined expressed PECAM- 1 at postnatal stages, in a cell population with the size and granularity selected by 1G11 ECs line. However, this expression was at different levels, perhaps, correlating with the vascular density. Besides, the cell adhesion molecule expression differs from prenatal to postnatal developmental stages in a determined organ, and among the organs studied.
Sheibani described the expression of several PECAM-1 isoforms in a developmentally regulated fashion, by RT-PCR analysis of RNA isolated from different tissues and ECs, in the early mouse embryo (E12) and postnatal mouse tissue (E21) (Sheibani et al., 2000). Our results show the relationship between this expression and the cellular morphological changes that undergo the two cell populations detected by FACS.
The slightly differences between prenatal and postnatal stages observed in brain suspension could be related to a different process of differentiation required by the complex process of blood-brain barrier formation.
The different pattern of expression of PECAM-1 could reflect potential organ differentiation pathways by soluble factors or via cell-cell interaction, which leads to the particular phenotype of organ endothelium.
Acknowledgments
This research was supported by grants from Buenos Aires University and CONICET. Rosa Wainstok is a Career Researcher from CONICET.
References
1. Baldwin SH, Shen HM, Yan HC, De Lisser HM, Chung A, Mickanin C, Trask T, Kirshbaum NE, Newman PJ, Albeda SM, Buck CA (1994). Platelet endothelial cell adhesion molecule-1 (PECAM-1): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development, 120: 2539-2553. [ Links ]
2. Calabrese GC, Fernandez de Recondo M, Recondo E, Wainstok de Calmanovici R (1998). Platelet endothelial cell adhesion molecule-1 expression during mouse postimplantation development. Cell Mol Biol, 44(3): 537-541. [ Links ]
3. De Lisser HM. Chilkotowsky J, Yan HC, Daise ML, Buck CA, Albeda SM (1994). Deletions in the cytoplasmic domain of plateletendothelial cell adhesion molecule-1 (PECAM-1) result in changes in ligand binding properties. J Cell Biol 124: 195-203. [ Links ]
4. Dong QG, Brnasconi S, Lostaglio S, Wainstok de Calmanovici R, Martin-Padura I, Brevario F, Garlanda C, Ramponi S, MantovanI A, Vecchi A (1997). A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterios Throm and Vas Biol 17(8): 1599-1604. [ Links ]
5. Lemberg A, Calabrese GC, Majowicz M, Peredo H, Scorticati C, Filinger E, Perazzo JC (1998). Prostanoid production in endothelial and Kupffer liver cells from monocrotaline intoxicated rats. Hum Exp Toxicol, 17: 564-569. [ Links ]
6. Loo D, Cotman C (1998). Primary and extended culture of embryonic mouse cells. In: Cell Biology: A Laboratory Handbook, Ed. Academic Press, New York, pp 65-72. [ Links ]
7. Newman PJ (1999). Switched at birth: a new family for PECAM-1. J Clin Invest, 103: 5-9. [ Links ]
8. Newman PJ (1997). Perspective Series: cell Adhesion in vascular biology. J Clin Invest, 99: 3-8. [ Links ]
9. Piedboeuf B, Gamache M, Frenette J, Horowitz S, Baldwin H (1998). Increased endothelial cell expression of platelet-endothelial cell adhesion molecule-1 during hyperoxic lung injury. Am J Respir Cell Mol Biol, 19(4): 543-553. [ Links ]
10. Risau W (1995). Differentiation of endothelium. FASEB, 9: 926- 933. [ Links ]
11. Sheibani N, Sorenson C, Frazier WA (2000). Differential modulation of chaderin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol Biol Cell, 11: 2793-2802. [ Links ]
12. Springer TA (1994). Traffic signals for limphocyte recirculation and leucocyte emigration: the multistep paradigm. Cell 76: 301-314. [ Links ]
13. Sun J, Paddock C, Shubert J, Zhang H-B, Amin K, Newman PJ, Albelda SM (2000). Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci, 113: 1459-1469. [ Links ]
14. Vecchi A, Garlanda C, Lampugnani MG, Resanti M, MatteuccI C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E (1994). Monoclonal antibodies specific to endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol, 63: 247-254. [ Links ]
15. Yan HC, Baldwin S, Sun J, Buck C, Albelda S, De Lisser H (1995). Alternative splicing of a specific cytoplasmatic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1). J Biol Chem, 270(40): 23672-23680. [ Links ]
Received on May 5, 2003
Accepted on May 14, 2004.














