Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.28 no.3 Mendoza Aug./Dec. 2004
Karyotype description of Pomacea patula catemacensis (Caenogastropoda, Ampullariidae), with an assessment of the taxonomic status of Pomacea patula
María Esther Diupotex-Chong1, Néstor J. Cazzaniga2, Alejandra Hernández-Santoyo3, and José Miguel Betancourt-Rule4
1 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Circuito Exterior, Ciudad Universitaria, Coyoacán 04510. México, D.F.
2 Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur. San Juan 670, 8000 Bahía Blanca. Argentina.
3 Universidad Autónoma Metropolitana Iztapalapa. Iztapalapa 09340. México, D.F.
4 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510. México, D.F.
Address correspondence to: Dra. María Esther Diupotex- Chong. Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Circuito Exterior, Ciudad Universitaria, Coyoacán 04510. MEXICO, D.F.
FAX: (+52-55) 616 0847. E-mail: medc@mar.icmyl.unam.mx
ABSTRACT: Mitotic chromosomes of the freshwater snail Pomacea patula catemacensis (Baker 1922) were analyzed on gill tissue of specimens from the type locality (Lake Catemaco, Mexico). The diploid number of chromosomes is 2n = 26, including nine metacentric and four submetacentric pairs; therefore, the fundamental number is FN = 52. No sex chromosomes could be identified. The same chromosome number and morphology were already reported for P. flagellata, i.e., the other species of the genus living in Mexico. The basic haploid number for family Ampullariidae was reported to be n = 14 in the literature; so, its reduction to n = 13 is probably an apomorphy of the Mexican Pomacea snails. Lanistes bolteni, from Egypt, also shows n = 13, but its karyotype is much more asymmetrical, and seems to have evolved independently from P. flagellata and P. patula catemacensis. The nominotypical subspecies, P. patula patula (Reeve 1856), is a poorly known taxon, whose original locality is unknown. A taxonomical account is presented here, and a Mexican origin postulated as the most parsimonious hypothesis.
Key words: apple snails, chromosomes, taxonomy, phylogeny, Mexico.
Introduction
Apple snails (family Ampullariidae) constitute a well-defined monophyletic group of freshwater mollusks, its members sharing more than 20 synapomorphies (Berthold, 1991; Bieler, 1993) within the Caenogastropoda Architaenioglossa (in the sense of Ponder and Lindberg, 1997). Most species of this family live in tropical and subtropical ecosystems of the Southern Hemisphere across Africa, Asia and America. Pomacea Perry 1810 is the most representative American genus, with 117 not yet synonymized nominal species, though the real number of species is probably near 50 (Berthold, 1989; Cowie and Thiengo, 2003).
Just a few species of Pomacea live to the north of 15ºN latitude and only two species are at present recognized in Mexico, namely, the widespread Pomacea flagellata (Say 1827) and the strictly endemic P. patula catemacensis (Baker 1922). The latter (locally named “tegogolo”) shows considerable variation among individuals both in size and color of the shell; these organisms are suitable models for genetic research and breeding experiments, due to their short life cycle, high hatching rate and number of eggs, and easy rearing (Martínez, 1989). They are also an important food resource in the area of Lake Catemaco (Veracruz-Llave State, Mexico), which is its type and only known locality (Naranjo-García and García-Cubas, 1986).
Only a few species of Pomacea have been studied from a cytogenetic viewpoint, and most authors have reported a haploid number of 14 chromosomes (Brand et al., 1990; Kawano et al., 1990; Mercado-Laczkó and Lopretto, 1998), which is the basic figure for the family according to Choudhury and Pandit (1997). Mexican species of Pomacea seem to make an exception however, since P. flagellata has a haploid number of 13 (Diupotex-Chong, 1994), and the same figure has been reported also for P. patula catemacensis (Yam, 1986; Diupotex-Chong et al., 1997). Sharing this character may be meaningful within the context of the current hypotheses of relationships among the American Ampullariidae.
The aim of this study is to describe the karyotype of Pomacea patula catemacensis, and to analyze its significance in the context of the phylogeny and taxonomy of the genus. A preliminary analysis of the taxonomic status of Pomacea patula (Reeve 1856), is also included.
Materials and Methods
Sixty live specimens of Pomacea patula catemacensis were collected from Lake Catemaco (18º 24’ N - 95º 04’ W), and reared in the laboratory until used for cytogenetic analysis.
Chromosomes from gill tissue in mitotic metaphase were mounted following the techniques described by Kligerman and Bloom (1977). The animals were injected with 0.5 ml of a 0.075 M KCl hypotonic solution, and were injected again 2 hours later with 0.5 ml of colchicine (0.02%). Two hours after the second injection, gills were excised and chopped.
Tissue pieces were placed into bi-distilled water for 20-30 min and fixed in at least two changes of fixative 3:1 methanol-acetic acid (freshly mixed) for 24 hours at 4ºC. The tissues were then minced gently and placed in 60% acetic acid for 30 min to prepare a cell suspension, which was placed onto a clean slide with a capillary tube (75 mm x 1.2 mm), and heated at 60ºC.
Staining was performed with 5% Giemsa in 0.1 M phosphate buffer, pH 6.8, and mounted in Canada balsam. Observations were made and micrographs were taken with a Carl Zeiss microscope. Fresh slides of gonadal tissue showing diakinesis phases were also analyzed under a phase contrast microscope to confirm the haploid number.
The relative length of each chromosome was expressed as a percentage of the absolute length of each chromosome pair out of the total length of the chromosome complement. The centromeric index was calculated as a percentage of the length of the short arm out of the total length of the chromosome. The arm ratio was calculated as the quotient long arm length/ short arm length (Q/P), and the chromosomes classified according to the terminology of Levan et al. (1964), i.e., chromosomes are named metacentric when they have a mean arm ratio of up to 1.7; submetacentric up to 3.0; subtelocentric up to 7.0; and telocentric over 7.0. Karyotype asymmetry was described following Stebbins (1950, 1971), and further assessed using the asymmetry indices A1 and A2 defined by Romero-Zarco (1986). A1 estimates the intrachromosomal asymmetry as  where n is the number of homologous chromosome pairs;
where n is the number of homologous chromosome pairs; ![]() is the average length for short arms in every chromosome pair, and
is the average length for short arms in every chromosome pair, and ![]() is the average length for long arms in every chromosome pair. A2 depicts the interchromosomal asymmetry as the ratio of the standard deviation to the mean length of the chromosomes. Karyotype asymmetry was compared with data published by previous authors on species of Ampullariidae.
is the average length for long arms in every chromosome pair. A2 depicts the interchromosomal asymmetry as the ratio of the standard deviation to the mean length of the chromosomes. Karyotype asymmetry was compared with data published by previous authors on species of Ampullariidae.
The presence of sex chromosomes was searched by looking for morphologically different heterosomes and/or heteropicnotic chromosome regions (Óstergren, 1950).
Results
Figure 1 shows a mitotic metaphase plate of gill tissue of Pomacea patula catemacensis, with 13 pairs of chromosomes, nine of which are metacentric and four submetacentric (Table 1). Diakinesis phase observed in squashes of fresh gonadal tissue confirmed the haploid number n = 13. Figure 2 shows the karyotype arranged by decreasing chromosome size; it reveals balanced size and form values, for both the P and Q arms. According to their form and architecture, the chromosomes evidenced a clear homologous setup by their relative length and centromeric index values. The fundamental number was NF = 52. Figure 3 depicts the ideogram constructed from the relative length and centromeric index values.

FIGURE 1. Metaphase chromosome spread of Pomacea patula catemacensis (Baker 1922). Scale bar = 5 mm.
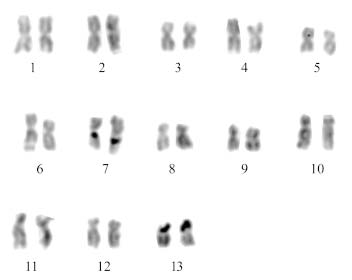
FIGURE 2. Representative karyotype of Pomacea patula catemacensis (Baker 1922).

FIGURE 3. Ideogram of Pomacea patula catemacensis chromosomes.
TABLE 1. Relative chromosome lengths and arm ratios in Pomacea patula catemacensis, from n = 15 representative metaphases. ![]() = mean length of the short arm (mm);
= mean length of the short arm (mm); ![]() = mean length of the long arm (mm); SD = standard deviation; TL = absolute total length
= mean length of the long arm (mm); SD = standard deviation; TL = absolute total length ![]() ; CI = centromeric index
; CI = centromeric index ![]() ;
; ![]() = mean relative length:
= mean relative length:![]() ;
; ![]() = mean arm ratio:
= mean arm ratio:![]() ; m = metacentric chromosomes; sm = submetacentric chromosomes.
; m = metacentric chromosomes; sm = submetacentric chromosomes.
Karyotype asymmetry corresponds to the category A2 of Stebbins (1971), since the ratio of the largest to the smallest chromosome in the karyotype was 1.74, and only one out of the 13 chromosomes (8%) showed an arm proportion <2:1. The intrachromosomal asymmetry index (A1) was 0.29, while the interchromosomal index (A2) was 0.17.
Sex chromosomes were not detected in our material, by observation neither of heterosomes, nor of heteropycnotic elements.
Discussion
Family Ampullariidae includes 13 genus-group named taxa; their phylogenetic relationships were analyzed by Berthold (1989, 1991) and Bieler (1993). Current hypotheses of ancestry advocate that all Neotropical genus-group taxa (namely, Asolene d’Orbigny 1837, Effusa Jousseaume 1889, Felipponea Dall 1919, Marisa Gray 1824, Pomacea Perry 1810, Pomella Gray 1847, and Surinamia Clench 1933) constitute a single monophyletic group, whose sister group is genus Pila Röding 1798, from Africa and Asia.
All the cytogenetically studied species of Pila have a haploid number of n = 14. This was the figure reported for Pila ovata (Oliver 1804) from Egypt (Lufty and Demian, 1965), and Pila virens (Lamarck 1819) from India (Ramamoorthy, 1967, cited by Choudhury and Pandit, 1997); no details on chromosome morphology were given in either case. A set of nine metacentric and five submetacentric chromosomes was described for Pila globosa (Swainson 1820) from India by Choudhury and Pandit (1997), who consider n = 14 to be the basic haploid number for the family. The same was reported for the Neotropical snail Marisa cornuarietis introduced into Egypt (Lufty and Demian, 1965), for Pomacea sp. from Southern Brazil (Kawano et al., 1990), and for Pomacea canaliculata from both native realm in South America and from Japan, where it was introduced in the eighties (Brand et al., 1990; Mercado-Laczkó and Lopretto, 1998). Instead, the two Mexican species of Pomacea have n = 13 (Yam, 1986; Diupotex-Chong, 1994; Diupotex-Chong et al., 1997; this study), which may represent an apomorphic condition within the American clade.
Berthold (1991) concluded that Marisa is the sister group of Pomacea sensu lato, i.e., Pomacea (Pomacea) + Pomacea (Effusa). On the other hand, Bieler’s (1993) strict consensus tree shows Marisa and Effusa as a clade that shares a more ancient ancestor with Pomacea s.s. Regrettably, there is no information on the karyotype of most ampullariids, and so we can only speculate that the coincidence of n = 13 in the two species of Pomacea living in Mexico may be a synapomorphy of the representatives living in the northern range of the genus. This hypothesis needs to be tested by studying the karyology of P. paludosa (Say 1829), P. cubensis (Morelet 1849), and P. poeyana (Pilsbry 1927), i.e., the native species of Pomacea s.s. that lives further northwards.
Shell morphology is often influenced by environmental conditions, geographic barriers, age of the organisms, and other factors affecting adaptation and selection (Inaba, 1961; Burch, 1960, 1967). Other sources of information may be enlightening, although incompatibility of specific delimitations based on different kinds of data are frequent. For example, while trying to characterize the species of Pomacea introduced into Thailand, Keawjam and Upatham (1990) demonstrated that some organisms with different shell or anatomic features may produce similar genetic patterns, whereas individuals that have almost identical morphology differ genetically. Number and morphology of the chromosomes may provide specific, crucial evidence for solving some taxonomic problems (White, 1973).
Brand et al. (1990) studied the chromosomes of a Pomacea species introduced into Japan, where it became an agricultural pest. The specimens were identified as Pomacea canaliculata (without any description, taxonomically useful illustration, or specific collection locality). A diploid chromosome formula of 20 m + 6 sm + 2 st was described for females, and 19 m + 7 sm + 2 st for males. Based on this limited information, and in the absence of other judgment resources, we conclude that the Japanese specimens were probably closer to the Brazilian specimens studied by Kawano et al. (1990) than to the Buenos Aires P. canaliculata.
Probably more than one closely related species of Pomacea were introduced into Japan, including both P. canaliculata and P. lineata (Cowie, 2002). Whichever their taxonomic relationships, the presence or absence of a subtelocentric pair in the diploid complement may be an argument for recognition, provided the constancy of this character is confirmed by further studies.
Karyotypes with a higher proportion of metacentric chromosomes, as shown by Pomacea patula catemacensis, are probably primitive, and show relative chromosome stability (White, 1951, 1978).The intrachromosomal asymmetry index is almost constant in the [Pila + Neotropical apple snails] clade (A1= 0.27- 0.31), i.e., five out of the six studied Ampullariid taxa (Fig. 4). The lowest value was calculated for an unidentified species from São Paulo (Brazil), probably Pomacea lineata (Spix 1827) whose chromosome formula is 9 m + 4 sm + 1 st (Kawano et al., 1990), while the highest asymmetry within this genus corresponds to Pomacea canaliculata (Lamarck 1822) from Buenos Aires Province (Argentina), with 11 metacentric and 3 submetacentric chromosomes (Mercado- Laczkó and Lopretto, 1998).

FIGURE 4. Karyotype asymmetry (as defined by Romero-Zarco, 1986) in six species of Ampullariidae. A 1 , intrachromosomal asymmetry index; A 2 , interchromosomal asymmetry index; Lb, Lanistes bolteni, Egypt (data after Yaseen et al., 1991); Pc, Pomacea canaliculata, Argentina (after Mercado-Laczk— and Lopretto, 1998); Pf, Pomacea flagellata, Mexico (after Diupotex-Chong, 1994); Pg, Pila globosa, India (after Choudhury and Pandit, 1997); Ppc, Pomacea patula catemacensis, México (this study); Psp, Pomacea sp., Brazil (after Kawano et al., 1990).
The sister group of the clade formed by Pila plus the Neotropical forms is the African, hyperstrophic genus Lanistes sensu lato (i.e., Lanistes + Plesiolanistes + Pseudoceratodes). Only one species of this group, identified as Lanistes bolteni from Egypt, has been studied from a karyological viewpoint, with conflicting results. While Lufty and Demian (1965) reported n = 14, without details on chromosome morphology and measurements, Yaseen et al. (1991) reported n = 13, with a chromosome formula 8 m + 2 st + 3 t. The presence of two subtelocentric and three telocentric chromosomes in Lanistes reveals a much more specialized and asymmetrical condition, suggesting that the reduction of its chromosome number to 13 is a non-homologous event, independent from the reduction to n = 13 in the Mexican species.
Brand et al. (1990) described the existence of male heterosomes X-Y, one metacentric and one submetacentric, which determine sex in Pomacea canaliculata from Japan. Such a heterogamety was not detected by other authors, neither in Pomacea, nor in other ampullariid genera; no sex chromosomes were identified in any Mexican species of Pomacea either (Diupotex-Chong, 1994; this study). It is evident that sex determination in these snails still needs further research. Yusa and Suzuki (2003) mentioned that a polyfactorial system of sex determination may exist in Pomacea, although it is also possible that different species or populations have different mechanisms.
Preliminary assessment of Pomacea patula (Reeve 1856).
Identifying and delimiting ancient morphospecies is a difficult task, especially when a type locality has not been stated (Cazzaniga, 2002). Pomacea patula is a poorly known species, described solely on the basis of its shell outline; it was at first compared only to Ampullaria neritoides d’Orbigny 1835 on the grounds of having an oddly broad aperture. The latter species is now recognized as a synonym of Pomella megastoma (Sowerby 1825) (Jaeckel, 1927; Pilsbry, 1933; Hylton- Scott, 1958), and its superficial similitude with P. patula proved not to be of taxonomic bearing. The original publication of P. patula included no data on the origin of the material, not even the continent where the species lived. The species was later omitted from most catalogues and taxonomic works (e.g., Sowerby, 1909; Alderson, 1925). Furthermore, the name was pre-occupied by Ampullaria patula Lamarck 1804 (now placed in genus Globularia, Naticidae), and so, A. patula Reeve 1856 is a junior primary homonym. However, no nomenclatural act is here included, waiting for a further study of the case.
To state that his new subspecies catemacensis fitted in P. patula, Baker (1922) compared it to a couple of shells collected in Mexico and labeled as P. patula in the Academy of Natural Sciences of Philadelphia collection. He acknowledged that these shells and P. patula catemacensis belong to the Ampullaria ghiesbrechtii group, i.e., the “Pomacea flagellata complex” (Pain, 1964), which is now considered a single, extremely variable species extending from Mexico and Central America to the Magdalena drainage area, in northern Colombia (Bequaert, 1957; Branson and McCoy, 1963; Rangel-Ruiz, 1988). Our cytogenetic results do not conflict with the relatedness of catemacensis and flagellata, which also has a haploid complement of 13 metacentric and submetacentric chromosomes (Diupotex-Chong, 1994).
Pomacea patula has been also mentioned as living in Brazil (a quotation from a personal communication by B. Walker, in Baker, 1922: 39). However, this conclusion was very weakly founded. Dr. Walker compared Baker’s pencil-sketched figures to the Reeve’s published figure, and referred that he also had four South American lots labeled “patula”: two of them collected in “New Granada”, i.e., Colombia plus Panama, a region where it is feasible to find P. flagellata- like apple snails; he was at the time unable to trace the third lot, collected from the Amazon, while the fourth lot, supposedly coming from Brazil, was “dealer’s specimens, and know nothing of their history” (Walker in Baker, 1922: 39 footnote). Therefore, there was no well-documented evidence of the presence of P. patula in Brazil.
Besides, later reports on the ampullariids from northern South America and the Amazon region failed to include P. patula (e. g., Baker, 1930; Bequaert, 1925; Geijskes and Pain, 1957; Haas, 1949, 1951; Jaeckel, 1952; Jousseaume, 1889; Martens, 1873; Pain, 1950, 1952, 1956, 1957, 1960; Pilsbry, 1933), except for a single mention in a checklist of a mollusk collection from the Mato Grosso region (Lopes, 1957) with no description or other data supporting this identification. The presence of a representative of the Pomacea flagellata group in the Amazon basin would be fairly astounding, and so the identity of the Mato Grosso P. patula should be reassessed. The concurrence of flagellata-like shell features and our karyological data hint at a Mexican origin for P. patula patula (Reeve 1856) as the most parsimonious hypothesis.
Acknowledgments
We would like to thank M.S. Mario Alejandro Gómez Ponce for the collection of organisms, Dr. Manuel Uribe Alcocer for his analysis suggestions, and Lea Cazzaniga for revising and improving the English wording. NJC is a member of the Scientific Researchers Career of the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, Argentina.
References
1. Alderson EG (1925). Studies in Ampullaria. Cambridge (England), W. Heffer and Sons. xx + 1-102. [ Links ]
2. Baker HB (1922). The mollusca collected by the University of Michigan-Walker expedition in Southern Veracruz Mexico. I. Occas Pap Mus Zool Univ Michigan 106: 1-62. [ Links ]
3. Baker HB (1930). The Mollusca collected by the University of Michigan-Williamson expedition to Venezuela. Part VI. Occas Pap Mus Zool Univ Michigan 210: 1-94. [ Links ]
4. Bequaert JC (1925). Malacological notes from the Amazon river, Brazil. Nautilus 39: 1-5. [ Links ]
5. Bequaert JC (1957). Land and freshwater snails of the Selva Lacandona, Chiapas, Mexico. Bull Mus Comp Zool 116: 204- 227. [ Links ]
6. Berthold T (1989). Comparative conchology and functional morphology of the copulatory organ of the Ampullariidae (Gastropoda, Monotocardia) and their bearing upon phylogeny and palaeontology. Abhandl naturwiss Ver Hamburg (NF), 28: 141-164. [ Links ]
7. Berthold T (1991). Vergleichende Anatomie, Phylogenie und historische Biogeographie der Ampullariidae (Mollusca: Gastropoda). Abhandl naturwiss Ver Hamburg (NF), 29: 1-256. [ Links ]
8. Bieler R (1993). Ampullariid phylogeny - Book review and cladistic re-analysis. Veliger, 36: 291-299. [ Links ]
9. Brand EV, Yokosawa T, Fujio Y (1990). Chromosome analysis of apple snail Pomacea canaliculata. Tohoku J Agric Res 40: 81- 89. [ Links ]
10. Branson BA, McCoy CJ (1963). Gastropoda of the 1961 University of Colorado Museum Expedition in Mexico. Nautilus 76: 101-108. [ Links ]
11. Burch JB (1960). Chromosomes of Pomatiopsis and Oncomelania. Amer Malac Union Ann Rep 1959, 26: 15. [ Links ]
12. Burch JB (1967). Chromosomes of mollusks. Proc Symp Moll II Mar Biol Assoc India, 1966: 635- 686. [ Links ]
13. Cazzaniga NJ (2002). Old species and new concepts in the taxonomy of Pomacea (Gastropoda: Ampullariidae). Biocell 26(1): 71- 81. [ Links ]
14. Choudhury RE, Pandit RK (1997). Chromosomes of three prosobranch gastropods from Viviparidae, Pilidae and Cyclophoridae (Order: Mesogastropoda). Caryologia 50: 303-315. [ Links ]
15. Cowie RH (2002). Apple snails as agricultural pests: their biology, impacts and management. In: Molluscs as Crop Pests (Ed. G. M. Barker), CABI, Wallingford. [ Links ]
16. Cowie RH, Thiengo SC (2003). The apple snails of the Americas (Mollusca: Gastropoda: Ampullariidae: Asolene, Felipponea, Marisa, Pomacea, Pomella): Nomenclatural and type catalog. Malacologia 45: 41-100. [ Links ]
17. Diupotex-Chong ME (1994). Karyological analysis and "G" bands of Pomacea flagellata from southeast Mexico. Western Soc Malac, Annu Rep, 26: 5-7. [ Links ]
18. Diupotex Chong ME, Foster N, Rubio SA (1997). Some chromosomic and electrophoretic characteristics of the genus Pomacea (Gastropoda: Pilidae) from the southeastern Mexico. In: Joint Meeting of the American Malacological Union and the Western Society of Malacologists. Santa Barbara, June 22-27, 1997. (Abstract. [ Links ])
19. Geiskes DC, Pain T (1957). Suriname freshwater snails of the genus Pomacea. Studies on the Fauna of Suriname and other Guyanas 1: 41- 48, pls. 9-10. [ Links ]
20. Haas F (1949). Land- und Süsswasser-mollusken aus dem Amazonas-Gebiete. Arch Molluskenk 78: 149-156. [ Links ]
21. Haas F (1951). Remarks and descriptions of South American non-marine shells. Fieldiana, Zool 31: 503-545. [ Links ]
22. Hylton-Scott MI (1958). Estudio morfológico y taxonómico de los ampulláridos de la República Argentina. Rev Mus Arg Cienc Nat "B Rivadavia", Zool 3: 233-333. [ Links ]
23. Inaba A (1961). Cytotaxonomy of the Euthyneuran gastropods. Venus, Jap J Malac 24: 402- 413. [ Links ]
24. Jaeckel SH (1927). Die Mollusken der Müllegger’schen Brasilien-Expedition. Zool Anz 72: 129-139. [ Links ]
25. Jaeckel S (1952). Short review of the land and freshwater molluscs of the North-East states of Brazil. Dusenia 3: 1-10. [ Links ]
26. Jousseaume F (1889). Voyage de M. Eugène Simon au Venezuela (Décembre 1887-Avril 1888). Mollusques. Mém Soc Zool France 2: 232-259. [ Links ]
27. Kawano T, Gómez LC, Correa F MA (1990). Chromosomes of Pomacea sp. (Perry, 1811) (Mesogastropoda, Mollusca). Rev Brasil Genet 13: 675- 685. [ Links ]
28. Keawjam RS, Upatham ES (1990). Shell morphology, reproductive anatomy and genetic patterns of three species of apple snails of the genus Pomacea in Thailand. J Med Appl Malac 2: 45- 57. [ Links ]
29. Kligerman AD, Bloom SE (1977). Rapid chromosome preparations from solid tissues of fish. J Fish Res Bd Can 34: 266-269. [ Links ]
30. Levan AK, Fredga A, Sandberg A (1964). Nomenclature for centromeric position on chromosomes. Hereditas 52: 201- 220. [ Links ]
31. Lopes HS (1957). Relação dos moluscos coletados na excurção às zonas das estradas de ferro noroeste do Brasil e Brasil-Bolívia, nos estados de Mato Grosso, Brasil e Bolívia. Publ Avulsas Mus Nac, Rio de Janeiro 20: 43-44. [ Links ]
32. Lufty RG, Demian ES (1965). Studies on the chromosome numbers in the Ampullariidae (Gastropoda, Prosobranchiata). Proc Egypt Acad Sci 18: 34-49. [ Links ]
33. Martens E von (1873). Die Binnenmollusken Venezuelas. Festschr Feier 100-jähr Besteh Gesellsch naturforsch Freunde, Berlin: 157-225, pls. 1-2. [ Links ]
34. Martínez GT (1989). Contribución al estudio y cultivo del caracol de agua dulce Pomacea patula catemacensis (Mesogastropoda: Ampullariidae). Secretaría de Educación Pública, Instituto Tecnológico del Mar Boca del Río Veracruz. Reporte Técnico Pesquero, oficio 342, 58 pp. [ Links ]
35. Mercado-Laczkó AC, Lopretto EC (1998). Estudio cromosómico y cariotípico de Pomacea canaliculata (Lamarck, 1801) (Gastropoda: Ampullariidae). Rev Mus Arg Cienc Nat "B. Rivadavia", Hidrobiol 8: 15-20. [ Links ]
36. Naranjo-García E, García-Cubas A (1986). Algunas consideraciones sobre el género Pomacea (Gastropoda: Pilidae) en México y Centroamérica. An Inst Biol Univ Nal Autón Méx, Ser Zool 56: 603-606. [ Links ]
37. Óstergren G. (1950). Isopycnosis and isopycnotic, two new terms for use in chromosomes studies. Hereditas, 36: 511. [ Links ]
38. Pain T (1950). Pomacea (Ampullariidae) of British Guaiana. Proc Malac Soc, London 28: 63-74, pl. 6-8. [ Links ]
39. Pain T (1952). Notes on the Pomacea of Surinam, with special reference to Ampullaria sowerbyi Vernhout. Basteria 16: 30-32. [ Links ]
40. Pain T (1956). On a collection of Pomacea from Colombia, with description of a new subspecies. J Conch 24: 73-79. [ Links ]
41. Pain T (1957). Pomacea of the Sierra de Merida, Venezuela. J Conch 24: 175-176. [ Links ]
42. Pain T (1960). Pomacea (Ampullariidae) of the Amazon River system. J Conch 24: 421-432. [ Links ]
43. Pain T (1964). The Pomacea flagellata complex in Central America. J Conch 25: 224-231. [ Links ]
44. Pilsbry HA (1933). Zoological results of the Matto Grosso Expedition to Brazil in 1931. II. Mollusca. Proc Acad Nat Sci Philadelphia 85: 67-76. [ Links ]
45. Ponder WF, Lindberg DR (1997). Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zool J Linn Soc 119: 83-265. [ Links ]
46. Rangel-Ruíz RJ (1988). Estudio morfológico de Pomacea flagellata (Say, 1827) (Gastropoda: Ampullariidae) y algunas consideraciones sobre su taxonomía y distribución geográfica en México. An Inst Biol Univ Nac Autón México, Zool 58: 21-34. [ Links ]
47. Romero-Zarco C (1986). A new method for estimating karyotype asymmetry. Taxon 35: 526- 530. [ Links ]
48. Sowerby GB (1909). Notes on the family Ampullariidae, with a list of species, varieties, and synonyms, also descriptions of four new species. Proc Malac Soc London 8: 345-362. [ Links ]
49. Stebbins GL (1950). Variation and evolution in plants. Columbia Univ Press, New York. pp 643. [ Links ]
50. Stebbins GL (1971). Chromosomal evolution in higher plants. Edward Arnold Ltd., London. [ Links ]
51. White MJD (1951). Citología animal y evolución. Buenos Aires, Espasa Calpe Argentina, pp 81- 107 and 179- 269. [ Links ]
52. White MJD (1973). Animal cytology and evolution. Cambridge Univ Press, Cambridge. 961 pp. [ Links ]
53. White MJD (1978). Chain processes in chromosomal speciation. Syst Zool 27: 17- 26. [ Links ]
54. Yam KE (1986). Análisis cariológico de la especie Pomacea patula catemacensis (Baker, 1922). Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México. 45 pp (unpublished). [ Links ]
55. Yaseen AE, Ebaid A-B M, Kawashti IS (1991). Studies on the chromosomal analysis and shell measurements of two species, Bellamya unicolor and Lanistes bolteni (Prosobranchiata: Gastropoda). Cytologia 56: 503-509. [ Links ]
56. Yusa Y, Suzuki Y (2003). A snail with unbiased population sex ratios but highly biased brood sex ratios. Proc R Soc Lond B 270: 283-288. [ Links ]
Received on October 27, 2003.
Accepted on July 28, 2004.














