Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.28 n.3 Mendoza ago./dic. 2004
RNA fingerprinting using RAP-PCR identifies an EBAF homologue mRNA differentially expressed in rat oviduct
Pablo A. Valdecantos, Martín E. Argañaraz, Carlos M. Abate, and Dora C. Miceli
Instituto Superior de Investigaciones Biológicas. INSIBIO (CONICET, Universidad Nacional de Tucumán), Chacabuco 461, (4000) S.M. de Tucumán, Argentina.
Address correspondence to: Dra. Dora C. Miceli. Instituto de Biología. Facultad de Bioquímica, Química y Farmacia. UNT. Chacabuco 461, (4000) Tucumán, ARGENTINA. FAX: (+54-381) 424 8025. E-mail: micelid@unt.edu.ar
Notes: The nucleotide sequences of Pr1, Pr2, Pr4, Pr7, Pr10, Pr11, Pr13 and Pr14 have been submitted to the GenBank and assigned the following accession numbers: Pr1: AW862648; Pr2: AF202265; Pr4: AW862643; Pr7: AW862645; Pr10: AF202267; Pr11: AF202266; Pr13: AW862646 and Pr14: AF202268.
ABSTRACT: As a step towards the identification of genes preferentially expressed in the oviduct during early rat embryo development, we isolated a cDNA fragment (Pr14) by using RNA arbitrarily primed PCR (RAP-PCR), being its expression restricted to oviduct and uterus; its mRNA is mainly expressed in oviduct during late luteal phase and early pregnancy. This fragment is 100% identical to a rat DNA sequence (Accession No. NW_047400) downstream the terminal exon of a Rattus norvegicus gene (Locus Link Accession No. LOC289316) similar to ebaf (endometrial bleeding-associated factor), a novel member of the Transforming Growth Factor superfamily. Northern analyses showed that this sequence hybridizes with 2.9 kb and 4.1 kb mRNAs in early pregnant rat oviducts. However, only the 4.1 kb mRNA was detected in the oviduct of non-pregnant rats, showing an increase from proestrus to diestrus. The expression of this oviduct-uterus specific mRNA suggests that the products of this gene may play a role in the oviductal reproductive process.
Key words: differential gene expression, oviduct, RNA fingerprint, cDNA
Abbreviations: bp, base pair(s); dNTP, deoxyribonucleoside triphosphate; ebaf, endometrial bleeding-associated factor; EST, expressed sequence tag; ORF, open reading frame; poly A, polyadenylation signal; RAPPCR, RNA arbitrarily primed PCR; TGF-beta, Transforming growth factor-beta; UTR, untranslated region.
Introduction
The mammalian oviduct undergoes certain physiological and biochemical modifications to provide a suitable environment for pregnancy. These changes are partly induced by specific proteins synthesized in the oviductal epithelium in response to varying concentrations of plasma hormones, especially estrogen and progesterone (Buhi et al., 1997a). Different studies have disclosed temporal and regional differences in the steady-state levels of specific mRNAs and in the de novo protein synthesis and secretion by the oviduct (Sutton et al., 1984; Gandolfi et al., 1989; Murray, 1993; Buhi et al., 2000). Proteins in the oviductal fluid provide the best microenvironment for embryo development (Murray, 1995; Xu et al., 2001) but the way in which this is accomplished is not completely known. An oviduct- specific glycoprotein (Donnelly et al., 1991; Buhi et al., 2000), protease inhibitors (Buhi et al., 1997b), plasminogen activators (Jimenez-Diaz et al., 2002), plasminogen activator inhibitor 1 (PAI-1) and tissue inhibitors of metalloproteinases (TIMPs) (Kouba et al., 2000b; Gabler et al., 2001), cytokines (Srivastava et al., 1996), binding proteins (Eberhardt et al., 1999) and growth factors (Chegini et al., 1994) have been identified in mammalian oviducts. Although the role of these proteins in fertilization and embryo development remains elusive, there is growing evidence that the oviduct actively supports embryo development (Yeung et al., 1992; Conway-Myers, 1998; Kouba et al., 2000a).
Recent endeavors have been made to identify the preferentially expressed genes in the mammalian oviduct that facilitate the development of the embryo in vivo and/or in vitro (Chang et al., 2000; Lee et al., 2000; Lee et al., 2002). It is known that gene expression of the mouse oviduct changes during the reproductive cycle (Lee et al., 2000) and, in addition, it has been possible to identify up-regulated genes during the early embryo preimplantation period (Lee et al., 2002). However, current knowledge of the majority of regulated genes that mediate the oviduct function is limited. As a step towards the identification of preferentially expressed genes in the rat oviduct, we studied the Pr14 temporal expression in normal rat oviduct during the estrous cycle and the embryo preimplantation period. The cloned sequences could reveal underlying information necessary to analyze biological processes that control the function of the oviduct during the first stages of embryonic development.
Materials and Methods
Animals
Virgin female Rattus norvegicus (Wistar), housed in an air-conditioned room with lights on from 7 am to 9 pm, weighing 200-250 g and exhibiting regular fourday cycles were used. In order to obtain four-day pregnant rats, animals in proestrus were mated with fertile males of the same strain. Day 1 of pregnancy was defined as the one in which spermatozoa were found in the vaginal smear of paired animals on the following morning. Female animals were killed between 9 am and 10 am on day 4 of pregnancy and the oviducts were collected.
Ovariectomized animals were obtained by surgical extraction of the ovaries through bilateral flank incisions, avoiding injury to the oviduct. Fifteen days later the animals were sacrificed and their oviducts removed.
RNA preparation
Oviducts were excised from approximately eight rats in each group, frozen in liquid nitrogen, and stored at -70ºC until use. Total RNA for cDNA synthesis and Northern analysis was isolated by the Chomczynski and Sacchi method (1987). In order to prevent DNA contamination, all RNA isolated samples were DNase treated according to manufacturer specifications. Poly (A)+ RNA was obtained from total RNA using the PolyATract mRNA isolation system (Promega, Madison, WI). Total RNA for RT-PCR analysis was obtained using the SV Total RNA Isolation System (Promega, Madison, WI).
cDNA synthesis and polymerase chain reaction
RAP-PCR was performed as described by Yoshida et al. (1994), with slight modifications. Poly (A)+ RNA (10 ng) and 25 pmol of an arbitrarily chosen primer were maintained at 68ºC for 5 min in diethyl pyrocarbonate (DEPC)-treated water (10 ml) and then set on ice. A final volume of 25 ml was achieved by addition to the total RNA and specific primer mix of 15 ml of a reverse transcription reaction mixture leading to the following final concentrations of its components: 50 mM Tris- HCl, pH 8.3; 75 mM KCl; 3 mM MgCl2; 0.5 mM of each dNTPs; 10 mM DTT; 25 units of Rnasin Ribonuclease Inhibitor (Promega) and 200 units of Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV) (Promega). The first strand cDNA synthesis was carried out at 34ºC for 30 min; heat inactivation of MMLV reverse transcriptase was made at 94ºC for 2 min. The resulting cDNA was stored at -70ºC.
Second-strand cDNA synthesis was also initiated by arbitrary priming by adding 2 ml of the first-strand cDNA to the PCR reaction mix. PCR amplification was performed in a final volume of 25 ml containing 10 mM Tris-HCl, pH 9.0; 50 mM KCl; 0.1% Triton X-100; 2.5 mM MgCl2; 200 mM of each dNTP; 1 mM of the same arbitrary primer as the one used for the first-strand synthesis and 2.0 units of Taq DNA polymerase (Promega). After the first denaturation cycle (94ºC for 2 min), reactions were carried out for 38 cycles at 94ºC for 55 s, 34ºC for 1 min, and 72ºC for 1 min, with a 7 min extension period at 72ºC. Five arbitrary 10-mer primers were used: A1, CCCAAGGTCC; A9, CTAATGCCGT; B1, TCGAAGTCCT; B6, GTGACATGCC; B7, AGATGCAGCC.
cDNA sequencing and database analysis
Differentially amplified cDNA fragments were cut from the gel using a razor blade; the piece of agarose was placed in a microcentrifuge tube; 2 ml of the eluent were PCR amplified using the PCR protocol described above with the corresponding primer. The amplified material was checked against the initial arbitrarily primed reaction by running a second agarose gel and the products were cloned into the pGEM-T easy vector (Promega). Cloned fragments were sequenced at The BioResource Center, Cornell University, Ithaca, NY. The sequences obtained were compared with databases from the National Center for Biotechnology Information web server (http://www.ncbi.nlm.nih.gov) using the BLAST algorithm (Altschul et al., 1990).
Northern blot analysis of RNA
Total oviductal RNA (10 mg) from early pregnant, ovariectomized animals and animals in proestrus, estrus and diestrus prepared as described above was denatured in the presence of glyoxal/DMSO and fractioned using 1.2% agarose gel electrophoresis (Sambrook et al.,1989). Ethidium bromide-stained rRNA bands were visualized (UV) to ensure that RNA degradation had not occurred and that equal amounts of RNA had been loaded into each lane (data not shown). After electrophoresis, RNA was transferred overnight to a modified charge nylon membrane (Sigma) by capillary blotting in 20 x SSC (3 M NaCl; 0.3 M sodium citrate, pH 7.0). After blotting, the membrane was baked for 2 h at 80ºC. It was then washed with 20 mM Tris-HCl, pH 8.0, at 65ºC to remove glyoxal from the RNA and prehybridized at 43ºC for 5 min in a prehybridization solution (0.25 M NaCl; 0.12 M Na2HPO4, pH 7.2; 1mM EDTA; 7% SDS; 50% formamide) (Ausubel et al., 1994). A biotinlabeled cDNA probe was added to a freshly prepared prehybridization solution (150 ml/cm2) and the membrane was hybridized at 43ºC overnight (about 16 h). The membrane was soaked in 2 x SSC and washed at room temperature on a rotating platform in 2 x SSC/ 0.1% SDS; 0.5 x SSC/0.1% SDS and finally in highstringency conditions at 65ºC in 0.1 x SSC/0.1% SDS for 15 min in each wash. Specific mRNAs were detected using the chemiluminescence reagent detection system, as described by BioLabs Inc. New England. Membranes were exposed to X-ray film (BioMax ML, Eastman Kodak, Rochester, NY). All the images were then captured with the Gel Doc 1000 image analyzer using the software Molecular Analyst 1.4.1 (BioRad).
Biotin-labeled probes were synthesized by PCR from cloned products (fragments Pr1, Pr2, Pr4, Pr13 and Pr14) using the T7-SP6 oligonucleotide primer set. b-actin mRNA was also examined to standardize for equal loading of RNA samples. Membranes were rehybridized after removing probes by washing in 75% formamide; 0.2 x SSC and 0.5% SDS for 1 h at 65ºC.
Semiquantitative RT-PCR
Total RNA (1 mg) from early pregnant and ovariectomized rat oviducts, and from uterus, ovary, kidney, muscle, intestine and liver from early pregnant rats, was reverse transcribed using oligo dT and M-MLV Reverse Transcriptase (Promega) in a 25 ml volume reaction. Reverse Transciptase was inactivated at 94ºC for 2 min and the RT sample was diluted to 50 ml with water; 1 ml of each sample was analyzed by PCR for Pr2, Pr4, Pr7, Pr10, Pr11 and Pr14 fragments with specific primer pairs. Primers and expected product sizes are as follows: Pr2-A (5’- CCTGCACTCTGGTTCAGTTCC -3’) and Pr2-B (5’- CTGCGACACAATGCTATACTGG -3’), 417 bp product; Pr4- A (5’- TCTGTAGAATGAATCCCTGCAA -3’) and Pr4-B (5’- TTGTGTCCAGCAAAGAATGC -3’), 397 bp product; Pr7- A (5’ CCTGAGACCCATGAAGTGGT -3’) and Pr7-B (5’- GCTCTCCTGGTCCATCTCAC -3’), 350 bp product; Pr10- A (5’- CGGGACATAATAGAAGCAGTGG -3’) and Pr10-B (5’- AGCATAAGTCAGTGGCTACCG -3’), 394 bp product; Pr11-A (5’- TTCAGCCACGAGGAAGAACT -3’) and Pr11- B (5’- CCCTGGACTTCCTGTTCAAG -3’), 349 bp product; Pr14-A (5’- TTACAAAGAGCTGCAGAGGGC -3’) and Pr14-B (5’- CACGTCACTCCAGTAGGACACC -3’), 349 bp product. The b-actin primer sequences target a region of the b-actin cDNA near the 5’ end of the molecule and result in an amplified fragment of 243 bp. The sequences are: 5’- CGTGGGCCGCCCTAGGCACCA -3’ for the upstream primer, and 5’- TTGGCCTTAGGGTTCAGGGGGG -3’ for the downstream primer; the primer pair spans an intron, which helps distinguish mRNA reverse-transcribed to cDNA from contaminating genomic DNA, which would generate a second PCR product larger than the one expected (Nudel et al., 1983; Watson et al., 1992).
b-actin amplification was as follows: 94ºC for 1 min; 19 cycles of 94ºC for 30 s, 58ºC for 30 s, and 72ºC for 1 min; with a 5 min extension period at 72ºC. The number of cycles for the other PCR reactions were: 28 for Pr2; 34 for Pr4; 32 for Pr7; 33 for Pr10; 34 for Pr11; 23 for Pr14; annealing temperatures were: 58ºC for Pr2, Pr10 and Pr14; 51ºC for Pr4; 52ºC for Pr7 and Pr11. Annealing temperatures were chosen on the basis of the specific primer sequences and amplification efficiency; the number of cycles for each primer set was chosen so that the amplified product would be in the linear range of amplification (not shown). RT-PCR samples were analyzed by 2% ethidium bromide-stained agarose gel electrophoresis.
RT-PCR assay for the identification of the Pr14 template strand
Two cDNA reactions were performed, one with primer Pr14-A and the other with primer Pr14-B. Total RNA (1 mg) from early pregnant rat oviduct and 25 pmol of each one of the two specific primers were maintained at 70ºC for 5 min in 10 ml of DEPC treated water and then put on ice. 15 ml of a reverse transcription reaction mixture to give a 25 ml final volume and concentration of the following components: 50 mM Tris-HCl, pH 8.3; 75 mM KCl; 3 mM MgCl2; 0.5 mM of each dNTP; 10 mM DTT, 25 units of Rnasin Ribonuclease Inhibitor (Promega) and 200 units of M-MLV Reverse Transcriptase (Promega) was added to total RNA and the specific primer mix. First-strand cDNA synthesis was carried out at 42ºC for 60 min; Reverse Transcriptase was heat inactivated at 94ºC for 2 min and the RT sample was diluted with water to 50 ml. The resulting reverse transcribed products were stored at -70ºC.
Each cDNA reaction product (1 ml) was PCR amplified with both Pr14-A and Pr14-B specific primers as follows: after the first denaturation cycle at 94ºC for 1 min, PCR reactions consisted of 25 cycles of 94ºC for 30 s, 58ºC for 30 s, and 72ºC for 50 s, with a 7 min extension period at 72ºC. RT-PCR samples were analyzed using 2% ethidium bromide-stained agarose gel electrophoresis.
Results
Detection of oviduct-specific transcripts by RNA fingerprinting
First-strand cDNA was synthesized with M-MLV Reverse Transcriptase using an arbitrarily selected primer (10-mer) as described in Materials and Methods. Reverse-transcribed cDNA fragments from poly (A)+ RNA isolated from pregnant and ovariectomized rat oviducts were used as templates for PCR amplification, with the same primer used for first-strand synthesis. To establish the most effective fingerprinting conditions, different assay conditions were examined, including magnesium and cDNA concentrations and annealing temperature. The optimum magnesium concentration was 2.5 mM. The resulting fingerprint patterns were highly reproducible using agarose gel electrophoresis followed by simple ethidium bromide staining (Fig. 1). We recovered from gels twelve specific PCR fragments differentially amplified. Lengths of Pr fragments were 500-1,500 bp. In this paper, we describe the analysis of eight of the differentially amplified fragments that were present in all the experiments carried out (Pr1, Pr2, Pr4, Pr7, Pr10, Pr11, Pr13 and Pr14) that were cloned and sequenced as indicated in Materials and Methods.

FIGURE 1. Representative profiles of RAP-PCR of RNA from early pregnant (Pr) and ovariectomized (Ovx) rat oviduct, subjected to 1.0 % (w/v) agarose gel electrophoresis. Five primers were used for PCR reactions. In A and B, these primers are indicated as A1, A9, B1, B6 and B7 (see Materials and Methods), at the top of the profiles. Arrows indicate the differentially amplified cDNA fragments. Positions and sizes of the components of a 100 bp DNA ladder (Promega) are indicated in the first and last lines of each profile.
Oviductal mRNA analysis by Northern blot hybridization and RT-PCR
Northern blot and RT-PCR analyses were used to verify that differentially displayed cloned cDNA fragments were differentially expressed in early pregnant and ovariectomized rat oviducts. Pr1, Pr2, Pr4, Pr13 and Pr14 fragments were selected for Northern blot hybridization assays. Biotinylated probes were generated by PCR from the cloned product. The mRNA of b-actin was determined in order to standardize the equal loading of RNA samples. The Pr1 and Pr13 probes detected 0.96 kb and 2.4 kb mRNAs respectively in both early pregnant and ovariectomized rat oviducts. In early pregnant oviducts, two hybridization signals of about 2.9 kb and 4.1 kb were obtained with the Pr14 probe; both signals were absent in ovariectomized oviducts (Fig. 2A). No hybridization signals were detected in samples from either early pregnant or ovariectomized oviducts when the Pr2 and Pr4 probes were used (not shown). On the contrary, when reevaluating the expression of these fragments by RT-PCR, a sensitive technique capable of detecting small amounts of transcript, it was possible to detect mRNA fragments amplified by specific Pr2 and Pr4 primers. On the other hand, coincidentally with Northern blot, the differential expression of Pr14 in oviducts from early pregnant rats was confirmed by RTPCR (Fig. 2B). The expression of other fragments (Pr7, Pr10, Pr11) in oviducts from early pregnant and ovariectomized animals was also evaluated by this technique. Neither early pregnant nor ovariectomized oviducts showed significant differences in the expression levels of these fragments.
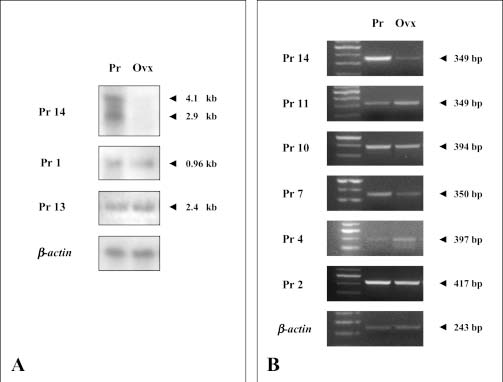
FIGURE 2. Verification of the expression of isolated cDNAs from RAP-PCR. A: Northern blot analysis of total oviductal RNA (10 mg) from early pregnant (Pr) and ovariectomized (Ovx) rats. The membrane was hybridized with biotin-labeled cDNA probes from clones Pr14, Pr1 and Pr13. mRNA sizes were determined by comparison with an RNA ladder (Promega). B: Semiquantitative RT-PCR of Pr2, Pr4, Pr7, Pr10, Pr11 and Pr14 from Pr and Ovx RNA samples as described in Materials and Methods. RNA samples in A and B were normalized using the house-keeping b-actin gene.
In each cDNA sample, b-actin expression (using primers described in Materials and Methods) was used as an internal control and as a means of detecting contaminating genomic DNA. No amplified b-actin genomic DNA product was observed in any of the RTPCR assays (Fig. 2B).
Pr14 expression in normal cycling rat oviducts and other organs
Pr14 expression was also evaluated by Northern hybridization in rat oviducts at different stages of the sexual cycle. 4.1 kb mRNA was detected during diestrus but with lower expression levels than the one observed in oviducts from pregnant rats when compared with the actin mRNA levels; the 2.9 kb hybridization signal was undetectable in the cycling rat oviducts (Fig. 3A).
The expression pattern of Pr14 in different organs of early pregnant rats was studied by RT-PCR using the specific pair of primers for Pr14. The specific 349 bp RT-PCR product was detected in uterus, but not in ovary, kidney, muscle, intestine or liver (Fig. 3B).

FIGURE 3. Pattern of expression of Pr14. A: Northern blot analysis of total oviductal RNA (10 mg) from normal cycling rats in proestrus (P), estrus (E), and diestrus (D). The membrane was hybridized with biotin-labeled cDNA probe from Pr14. B: semi-quantitative RT-PCR of Pr14 clone in early pregnant rat oviducts (Pr), rat uterus (U), ovary (O), liver (L), kidney (K), intestine (I) and muscle (M). Rat tissue total RNA was subjected to RT-PCR, as described in Materials and Methods. RNA samples were normalized and sizes were determined, as indicated in figure 2.
Identification of the Pr14 template strand
Since Pr14 is a RAP-PCR product that contains terminal repeats and we were not able to confirm the presence of a functional ORF, the knowledge of the template strand of Pr14 was of great interest for us. In order to determine the sense strand of the Pr14 clone, we designed a rapid RT-PCR based approach (Fig. 4A). Two cDNA reactions using total RNA from early pregnant rat oviduct as a template were performed, one with primer Pr14-A and the other with Pr14-B. PCR amplification of each cDNA product was performed using both Pr14-A and Pr14-B. In theory, only one of the two amplification reactions should generate a RT-PCR product of the expected size. Results are shown in Fig. 4B. The specific product was observed only in the cDNA obtained with primer Pr14-A, the antisense primer, which allowed the identification of the polarity of the natural RNA. This RT-PCR approach could be useful for the rapid identification of the sense strand of any uncharacterized Expressed Sequence Tag (EST) instead of the time consuming classical Northern blotting experiment with single strand probes generated from the cloned RAP-PCR product.

FIGURE 4. Template strand identification of Pr14. A, Schematic diagram of the RT-PCR approach. B, Agarose gel electrophoresis of amplified cDNA from pregnant rat oviduct total RNA. Positions and sizes of markers are indicated in Line 1. Line 2 depicts the PCR amplification of reverse transcription reaction products, using primer Pr14-A: 5' TTACAAAGAGCTGCAGAGGGC-3'. Line 3: PCR amplification of reverse transcription reaction products, using primer Pr14-B: 5'-CACGTCACTCCAGTAGGACACC-3.
Analysis of differentially expressed cDNA sequences
BLAST algorithms were employed to search the cDNA sequences of the cloned fragments for known genes and EST similarities in the GenBank/EMBL database.
Pr1 showed identity with the mouse ATPase subunit 6 mRNA (Accession No. AF093677). The Pr2 sequence is similar to the 4,614 bp Homo sapiens mRNA for KIAA1380 protein (Accession No. AB037801). Pr4 and Pr7 showed no matches with any of the database entries. The Pr10 fragment was homologous to a 4,039 bp cDNA of the human KIAA1585 protein (Accession No. AB046805). The sequence of Pr11 matched a Rattus norvegicus mRNA coding for the centrosomal protein CG-NAP (Accession No. AB071391). The cDNA sequence of Pr13 showed homology with the 2,073- nucleotide mRNA of the rat heat-shock cognate protein 70 (Hsc70).
Sequence homology searches and comparisons of the Pr14 sequence against the GenBank/EMBL databases found an identical sequence in the Rat genomic BLAST page (http://www.ncbi.nlm.nih.gov/genome/ seq/RnBlast.html), showing that Pr14 has a 100% identity to a 616 bp of the Rattus norvegicus chromosome 13 (Accession No. NW_047400). Data obtained from this sequence from the GenBank predicted a Rattus norvegicus gene between nucleotides 1,462,207 and 1,467,028 with four exons and a coding deducted sequence (CDS) of 1,101 bp coding for a 366 amino acids protein similar to the human endometrial bleedingassociated factor (Accession No. AAB53269).
Our results indicate that the Pr14 sequence is downstream the terminal exon between nucleotides 1,467,040 and 1,467,655 and upstream potential poly A signals at nucleotides 1,468,056; 1,468,060; 1,468,268; 1,469,985 and 1,470,372 of the NW_047400 sequence. These poly A signals are at 1,028; 1,032; 1,240; 2,957 and 3,344 nucleotides from the end of the CDS of the terminal exon.

FIGURE 5. Schematic representation (not drawn to scale) of the ebaf gene from the unordered rat chromosome 13 sequence (Accession No. NW_047400). Empty hollow rectangles indicate the coding deducted sequences (CDS). The full rectangle represents the Pr14 sequence. Triangles indicate potential poly A signals. Numbers indicate nucleotide positions of the beginning and the end of each CDS, Pr14 sequence, and potential poly A sites positions. Nucleotide 1 corresponds to START codon; nucleotide 4,464 corresponds to STOP codon.
Discussion
RNA fingerprinting using arbitrarily primed PCR (RAP-PCR) (Welsh et al., 1992) and differential display reverse transcription PCR (DDRT-PCR) (Liang and Pardee, 1992) allow the semiquantitative simultaneous comparison of the abundance of several hundred randomly sampled RNAs. RNA fingerprinting has been used to identify transcripts that are aberrantly regulated in human tumors (Liang et al., 1992), differentially expressed in neurons of different culture age (Li et al., 2002), in human common congenital heart defects (Sun et al., 2002), and in the identification of genes that are up regulated in rheumatoid synovial fibroblasts (Kullmann et al., 1999).
As demonstrated in this report, RAP fingerprinting can be used to identify transcripts that are differentially expressed in the oviduct of early pregnant rats. It allows the direct comparison of gene expression with the oviduct of ovariectomized rats used as an internal standard. Several fingerprint products from putative differentially expressed messages in the pregnant oviduct were detected. Although RAP-PCR is a rapid and convenient method for identifying differentially expressed genes, it generates a high level of false positives and is biased for high copy-number mRNA (Bertioli et al., 1995). The pattern of amplified products from the oviduct was obtained with high reproducibility in independent PCR experiments. Seven fingerprint PCR products (Pr1, Pr2, Pr4, Pr7, Pr10, Pr11 and Pr13) were detected in both early pregnant and ovariectomized oviducts. Pr1, Pr11 and Pr13 sequences were identical to known rat genes, but Pr2 and Pr10 share a high similarity with two human mRNA sequences coding for proteins with unknown functions not yet described in rats: Homo sapiens KIAA1380 mRNA (Accession No. AB037801) and Homo sapiens KIAA1585 mRNA (Accession No. AB046805) respectively. Although the Pr2 transcript was amplified by RT-PCR, it was not detectable in Northern blots using total RNA, indicating that this fragment might correspond to a low abundance gene type. The identities of Pr4 and of Pr7 are as yet unknown, since they showed no similarity to GenBank entries, although the presence of numerous STOP codons suggests that these fragments probably belong to a UTR region of mRNAs.
Two transcripts of about 2.9 kb and 4.1 kb detected by Northern hybridization as being differentially expressed in early pregnant rat oviducts at the preimplantational stage were identified with the Pr14 probe.
Further analyses during the estrous cycle showed that the 2.9 kb mRNA was virtually absent in cycling non-pregnant rat oviducts. Although the 4.1 kb mRNA was observed in cycling non-pregnant rat oviducts, the hybridization signal was weaker than in early pregnant ones. The 4.1 kb mRNA expression levels detected during proestrus gradually increased through estrus to diestrus, suggesting that this cDNA possibly corresponds to a gene expressed under steroids hormone influence.
Pr14 sequence comparison with the Rat Genomic database showed a 100% identity to the Rattus norvegicuschromosome 13 (Accession No. NW_047400) harboring a predicted four exon gene coding for a 366 amino acids protein similar to the human ebaf (AAB53269), a secreted protein member of the TGF-beta superfamily with two conserved domains: the TGF-beta propeptide, also known as latency associated peptide (LAP) in TGFbeta, and the TGF-beta domain, which is a multifunctional peptide that controls proliferation, differentiation, and other functions in many cell types.
As a first step towards the characterization of the Pr14 clone, we developed a rapid RT-PCR method using sense and antisense primers in the first-step cDNA synthesis, an approach that proved effective in the identification of the sense strand of the Pr14 clone. Results suggest that the Pr14 sequence is downstream the terminal exon of the rat ebaf homolog gene and belongs to its 3’ UTR. Potential poly A signals are downstream the Pr14 sequence at 1,028; 1,032; 1,240; 2,957 and 3,344 nucleotides from the end of the CDS of the terminal exon; taking into account that the predicted CDS of this gene is 1,101 bp in length, the sizes of the mRNAs could be 2,129; 2,133; 2,341; 4,058 and 4,445 nucleotides respectively. Interestingly, the sizes of the two transcripts of about 2.9 kb and 4.1 kb detected by Northern hybridization are in agreement with some of those calculated from the genomic sequence. As Pr14 is upstream to all the potential poly A signals, we hypothesized that the 2.9 kb and 4.1 kb transcripts could be originated by an alternative poly A site recognition mechanism (Cudry et al., 1999; Qu et al., 2002). In our scheme, the poly A site nearest to the end of the CDS of the terminal exon could be well read during early pregnancy (producing the 2.9 kb mRNA) but could also be skipped during the non pregnant stage (producing preferentially the 4.1 kb mRNA), although in low quantities when compared with the early pregnant stage. It is important to note that up to three mRNA species of the human ebaf in endometria have been detected (Tabibzadeh, et al. 2000). When checking the GenBank nucleotide sequence of the ebaf(Accession No. U81523), we found potential poly A sites that could generate the ebaf mRNA species reported in human. At present, it is known that certain UTRs of eukaryotic mRNAs play an important role in gene regulation and expression (Chen and Shyu, 1995; Qi and Pekala, 1999). The 3’ UTR of the rat mRNA harboring the Pr14 sequence could be involved in the regulation of the levels of this mRNA. The human ebaf expression in endometria has been reported to be confined to both the late secretory phase and during endometrial bleeding; the expression of this gene was absent in a host of normal tissues including lung, kidney, ovary, liver, colon, stomach, breast, lymph node, spleen and fallopian tube (Kothapalli et al., 1997). However, a weak expression of the 2.1 kb variant mRNA was observed in rectal, ovarian, and testicular tissues and the 2.1 and 2.5 kb mRNAs were observed in the pancreatic tissue (Tabibzadeh et al., 1997). It has also been reported that ebaf stimulates the production of human matrix metalloproteinases (MMPs) during menstruation and that progesterone controls both the expression of ebaf and its effect on these MMPs when combined with estradiol (Cornet et al., 2002).
To the best of our knowledge, this is the first report of ebaf expression in the oviduct. Taking into account that bovine ovarian granulosa- and theca-cells, oocytecumulus complex and oviductal epithelium synthesize growth factors and extra-cellular matrix-components such as matrix MMPs (Einspanier et al., 1999; Gabler et al., 2001) and that the expression pattern of Pr14 by RT-PCR has shown that Pr14 is expressed in the rat oviduct and uterus but in no other organ, this expression being hormonally regulated apparently by ovarian steroids, it seems probable that the corresponding gene should have a function in the oviductal reproductive process.
Acknowledgments
This study was supported partially by the National Research Council of Argentina (CONICET), National Promoting Scientific and Technological Agency (BID 1201/OC-AR PICT 08-05430) and the Research Council of the National University of Tucumán (CIUNT, grant 26/D122).
We thank Dr. M. Baigorí for kindly providing the arbitrary 10-base primers. We are also grateful to Dr. María Jimenez Díaz for technical assistance in the handling and ovariectomization of rats, and PhD student E.M. Roldán Olarte for help with Northern blot hybridization.
References
1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. J Mol Biol. 215: 403-410. [ Links ]
2. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994). Current Protocols in Molecular Biology. John Wiley and Sons, pp. 2.10.11-12. [ Links ]
3. Bertioli DJ, Schlichter UH, Adams MJ, Burrows PR, Steinbiss HH, Antoniw JF (1995). An analysis of differential display shows a strong bias towards high copy number mRNAs. Nucleic Acids Res. 23: 4520-4523. [ Links ]
4. Buhi WC, Alvarez IM, Kouba AJ (1997a). Oviductal regulation of fertilization and early embryonic development. J Reprod Fertil Suppl. 52: 285-300. [ Links ]
5. Buhi WC, Alvarez IM, Pickard AR, McIntush EW, Kouba AJ, Ashworth CJ, Smith MF (1997b). Expression of tissue inhibitor of metalloproteinase-1 protein and messenger ribonucleic acid by the oviduct of cyclic, early-pregnant, and ovariectomized steroidtreated gilts. Biol Reprod. 57: 7-15. [ Links ]
6. Buhi WC, Alvarez IM, Kouba AJ (2000). Secreted proteins of the oviduct. Cells Tissues Organs. 166: 165-179. [ Links ]
7. Chang HS, Cheng WT, Wu HK, Choo KB (2000). Identification of genes expressed in the epithelium of porcine oviduct containing early embryos at various stages of development. Mol Reprod Dev. 56: 331-335. [ Links ]
8. Chegini N, Zhao Y, McLean FW (1994). Expression of messenger ribonucleic acid and presence of immunoreactive proteins for epidermal growth factor (EGF), transforming growth factor alpha (TGF alpha) and EGF/TGF alpha receptors and 125I-EGF binding sites in human fallopian tube. Biol Reprod. 50: 1049-1058. [ Links ]
9. Chen CY, Shyu AB (1995). AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 20: 465-470. [ Links ]
10. Chomczynski P, Sacchi N (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 162: 156-159. [ Links ]
11. Conway-Myers BA (1998). Co-culture update: creating an embryotrophic environment in vitro. Semin Reprod Endocrinol. 16: 175-182. [ Links ]
12. Cornet PB, Picquet C, Lemoine P, Osteen KG, Bruner-Tran KL, Tabibzadeh S, Courtoy PJ, Eeckhout Y, Marbaix E, Henriet P (2002). Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloprotineases. J Biol Chem. 277: 42496-42504. [ Links ]
13. Cudry S, Froissart R, Bouton O, Maire I, Bozon D (1999). The 2.1-, 5.4- and 5.7-kb transcripts of the IDS gene are generated by different polyadenylation signals. Biochim Biophys Acta 1447: 35-42. [ Links ]
14. Donnelly KM, Fazleabas AT, Verhage HG, Mavrogianis PA, Jaffe RC (1991). Cloning of a recombinant complementary DNA to a baboon (Papio anubis) estradiol-dependent oviduct-specific glycoprotein. Mol Endocrinol. 5: 356-364. [ Links ]
15. Eberhardt DM, Jacobs WG, Godkin JD (1999). Steroid regulation of retinol-binding protein in the ovine oviduct. Biol Reprod. 60: 714- 720. [ Links ]
16. Einspanier R, Gabler C, Bieser B, Einspanier A, Berisha B, Kosmann M, Wollenhaupt K, Schams D (1999). Growth factors and extracellular matrix proteins in interactions of cumulus-oocyte complex, spermatozoa and oviduct. J Reprod Fertil Suppl. 54: 359-365. [ Links ]
17. Gabler C, Killian GJ, Einspanier R (2001). Differential expression of extracellular matrix components in the bovine oviduct during the oestrous cycle. Reproduction 122: 121-130. [ Links ]
18. Gandolfi F, Brevini TA, Richardson L, Brown CR, Moor RM (1989). Characterization of proteins secreted by sheep oviduct epithelial cells and their function in embryonic development. Development 106: 303-312. [ Links ]
19. Jimenez-Diaz M, Roldan M, Miceli DC (2002). Localization of plasminogen in the extracellular matrix of hamster eggs: exogenous activation by streptokinase. Mol Reprod Dev. 61: 528-535. [ Links ]
20. Kothapalli R, Buyuksal I, Wu SQ, Chegini N, Tabibzadeh S (1997). Detection of ebaf, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding. J Clin Invest. 99: 2342-2350. [ Links ]
21. Kouba AJ, Abeydeera LR, Alvarez IM, Day BN, Buhi WC (2000a). Effects of the porcine oviduct-specific glycoprotein on fertilization, polyspermy, and embryonic development in vitro. Biol Reprod. 63: 242-250. [ Links ]
22. Kouba AJ, Burkhardt BR, Alvarez IM, Goodenow MM, Buhi WC (2000b). Oviductal plasminogen activator inhibitor-1 (PAI-1): mRNA, protein, and hormonal regulation during the estrous cycle and early pregnancy in the pig. Mol Reprod Dev. 56: 378-386. [ Links ]
23. Kullmann F, Judex M, Ballhorn W, Justen HP, Wessinghage D, Welsh J, Yen TJ, Lang B, Hittle JC, McClelland M, Gay S, Scholmerich J, Muller-Ladner U (1999). Kinesin-like protein CENP-E is upregulated in rheumatoid synovial fibroblasts. Arthritis Res. 1: 71-80. [ Links ]
24. Lee K, Kwok K, Yeung W (2000). Suppression subtractive hybridization identifies genes expressed in oviduct during mouse preimplantation period. Biochem Biophys Res Commun. 277: 680-685. [ Links ]
25. Lee KF, Yao YQ, Kwok KL, Xu JS, Yeung WS (2002). Early developing embryos affect the gene expression patterns in the mouse oviduct. Biochem Biophys Res Commun. 292: 564-570. [ Links ]
26. Li Q, Li Z, Sun CX, Yu AC (2002). Identification of transcripts expressed under functional differentiation in primary culture of cerebral cortical neurons. Neurochem Res. 27: 147-154. [ Links ]
27. Liang P, Averboukh L, Keyomarsi K, Sager R, Pardee AB (1992). Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 52: 6966-6968. [ Links ]
28. Liang P, Pardee AB (1992). Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257: 967-971. [ Links ]
29. Murray MK (1993). An estrogen-dependent glycoprotein is synthesized and released from the oviduct in a temporal- and region-specific manner during early pregnancy in the ewe. Biol Reprod. 48: 446-453. [ Links ]
30. Murray MK (1995). Epithelial lining of the sheep ampulla oviduct undergoes pregnancy-associated morphological changes in secretory status and cell height. Biol Reprod. 53: 653-663. [ Links ]
31. Nudel U, Zakut R, Shani M, Neuman S, Levy Z, Yaffe D (1983). The nucleotide sequence of the rat cytoplasmic b-actin gene. Nucleic Acids Res. 11: 1759-1771. [ Links ]
32. Qi C, Pekala PH (1999). The influence of mRNA stability on glucose transporter (GLUT1) gene expression. Biochem Biophys Res Commun. 263: 265-269. [ Links ]
33. Qu X, Qi Y, Qi B (2002). Generation of multiple mRNA transcripts from the novel human apoptosis-inducing gene hap by alternative polyadenylation utilization and the translational activation function of 3' untranslated region. Arch Biochem Biophys. 400: 233- 244. [ Links ]
34. Sambrook J, Fritsch EF, Maniatis T (1989). Molecular Cloning: A laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 7.40-42. [ Links ]
35. Srivastava MD, Lippes J, Srivastava BI (1996). Cytokines of the human reproductive tract. Am J Reprod Immunol. 36: 157-166. [ Links ]
36. Sun G, Yuen Chan S, Yuan Y, Wang Chan K, Qiu G, Sun K, Ping Leung M (2002). Isolation of differentially expressed genes in human heart tissues. Biochim Biophys Acta 1588: 241-246. [ Links ]
37. Sutton R, Nancarrow CD, Wallace AL, Rigby NW (1984). Identification of an oestrus-associated glycoprotein in oviductal fluid of the sheep. J Reprod Fertil. 72: 415-422. [ Links ]
38. Tabibzadeh S, Kothapalli R, Buyuksal I (1997). Distinct tumor specific expression of TGFB4 (ebaf)*, a novel human gene of the TGFbeta superfamily. Front Biosci. 2: a18-25. [ Links ]
39. Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B (2000). Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab. 85: 2526-2536. [ Links ]
40. Watson AJ, Hogan A, Hahnel A, Weimer KE, Schultz GA (1992). Expression of growth factor ligand and receptor genes in the preimplantation bovine embryo. Mol Reprod Dev. 31: 87-95. [ Links ]
41. Welsh J, Chada K, Dalal SS, Cheng R, Ralph D, McClelland M (1992). Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res. 20: 4965-4970. [ Links ]
42. Xu JS, Cheung TM, Chan ST, Ho PC, Yeung WS (2001). Temporal effect of human oviductal cell and its derived embryotrophic factors on mouse embryo development. Biol Reprod. 65: 1481-1488. [ Links ]
43. Yeung WS, Ho PC, Lau EY, Chan ST (1992). Improved development of human embryos in vitro by a human oviductal cell co-culture system. Hum Reprod. 7: 1144-1149. [ Links ]
44. Yoshida KT, Naito S, Takeda G (1994). cDNA cloning of regeneration-specific genes in rice by differential screening of randomly amplified cDNAs using RAPD primers. Plant Cell Physiol. 35: 1003-1009. [ Links ]
Received on November 4, 2003.
Accepted on May 21, 2004.














