Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.29 n.1 Mendoza ene./abr. 2005
Fine structural analysis of the epithelial cells in the hepatopancreas of Palaemonetes argentinus (Crustacea, Decapoda, Caridea) in intermoult
Liliana G. Sousa1, Elena I. Cuartas2, and Ana María Petriella1, 3
1. Departamento de Ciencias Marinas.
2. Departamento de Biología, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata. Funes 3350. B7602AYL, Mar del Plata. Argentina.
3. CONICET.
Address correspondence to: Lic. Liliana G. Sousa. Departamento de Ciencias Marinas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata. Funes 3350. (B7602AYL) Mar del Plata, ARGENTINA. FAX: (+54-223) 475-3150. E-mail: lgsou@mdp.edu.ar
Abstract: The aim of this study is to describe the ultrastructure of the hepatopancreas of P. argentinus in intermoult. P. argentinus hepatopancreas was studied using standard TEM techniques. Each tubule consists of four cellular types: E (embryonic), F (fibrillar), R (resorptive) and B (blister like). E-cells have embryonic features and some of them were found in mitosis. F, R and B cells possess an apical brush border. F-cells have a central or basal nucleus, a conspicuous RER, and dilated Golgi cisternae. R cells show a polar organization of organelles in three areas: apical, with numerous mitochondria and sER tubules, a central area with the nucleus and RER, and a basal area containing a sER-like tubule system and mitochondria. B-cells were observed at different stages of their life cycle. In an early differentiation stage they comprise an apical endocytotic complex and Golgi vesicles. The fusion of endocytotic and Golgi vesicles originates subapical vacuoles. During maturation, a big central vacuole is formed by coalescence of subapical vacuoles. The central vacuole is eliminated by holocrine secretion. The ultrastructure suggests that F-cells synthesize proteins, Rcells storage nutrients and B-cells have a secretory or excretory function, and confirms the independent origin of F, B and R cells from the embryonic cells.
Keywords: Crustacea. Caridea. Hepatopancreas. Ultrastructure
Introduction
In crustacean, the hepatopancreas is the primary organ responsible of absorption and storage of ingested materials (Loizzi, 1971; Storch and Welsch, 1977; Vogt et al., 1989; Johnston et al., 1998). This organ is also involved in the synthesis of digestive enzymes and the detoxification of xenobiotics (Gibson and Barker, 1979; Icely and Nott, 1992; Vogt, 1994). It consists of one or more lobules formed by tubules with blind ends at the distal zone and open ends at the proximal zone. Tubules converge into a primary duct, which connects the organ with the pyloric stomach (Icely and Nott, 1992; Sousa and Petriella, 2000).
Hepatopancreas epithelium is composed of four main cell types: E (embryonic) cells, confined to the blind distal ends, and F (fibrilar), R (resorptive) and B (blisterlike) cells, distributed throughout the whole tubule with some variations according to the species (Icely and Nott, 1992; Petriella and Fonalleras, 1997; Sousa and Petriella, 2000). The organ undergoes histological and histochemical modifications in response to different physiological demands (moult, reproduction) (Al- Mohanna and Nott, 1989; Sousa and Petriella, 2001) and environmental changes (physicochemical factors, pollution). As a result, there is a correlation between the physiological condition of the organism and the hepatopancreas structure (Popescu-Marinescu et al., 1997). In hepatopancreas of Penaeus monodon exposed to pesticides, Vogt (1987) found disorganization of tubule morphology, epithelium autolysis, reduction of endoplasmic reticulum, and swollen mitochondria.
The prawn P. argentinus is widely distributed in the littoral region of Argentina, Paraguay, Uruguay and southern Brazil (Boschi, 1981; Morrone and Lopreto, 1995). This species lives in freshwater and brackish water streams and lagoons and plays an important trophic role in the environments in which it inhabits (Spivak, 1997; Collins, 1999). Some of these lagoons receive direct discharges of chemicals from terrestrial ecosystems, from which P. argentinus accumulates important amounts of organochlorine pesticides in its tissues (Gonzalez Sagrario et al., 1998). The present study is part of a project which aims to use the histological changes in this organ as indicators of environmental pollution. This work describes the fine structure and function of the different cell types (E, F, R and B) of P. argentinus hepatopancreas.
Materials and Methods
Intermoult adults of both sexes at sexual rest (Boschi, 1981), 0.100-0.200g in weight and 22.2-29.6 mm in total length, were collected from Sotelo stream, tributary of Mar Chiquita lagoon (Argentina, 38°S 55°W). This stream is a shallow creek (<1m in the channel) where pesticide concentrations are below the toxic levels and are not related to adverse biological effects (Miglioranza et al., 2003). The moult stage was determined by microscopic examination of the setae of the uropod exopodite, following the criteria established by Díaz et al. (1998).
The animals were placed on ice and the cephalothorax integument was removed. The hepatopancreas were dissected and placed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH= 7.2- 7.4) overnight at 4°C. They were postfixed for 1h in 1% OsO4. Later the material was dehydrated through an ethanol series and embedded in Spurr resin. Semithin sections (1mm) were stained with toluidine blue. Ultrathin sections were mounted on copper grids (400mesh) and stained with lead citrate and uranyl acetate. TEM images were obtained with a Hitachi HU 11C-1 transmission electron microscope.
Results
Each hepatopancreatic tubule is lined by a simple epithelium which consists of four cell types: E (embryonic), F (fibrillar), R (absorptive) and B cells (blister-like) (Fig. 1).

FIGURE 1. Cross section of a tubule. Detail of the specialized cellular types. B: B cells, F: F cells, R: R cells, v: vacuole (From Sousa and Petriella, 2000). H&E. X 1,000. Scale bar: 50 mm.
E-cells are located at the distal blind end of the tubules. They are cubical, lack of brush border and have a high nucleus to cytoplasm volume ratio, typical of embryonic cells (Fig. 2). The cytoplasm is homogeneous with few organelles and a poorly developed ER. Slight concentrations of mitochondria are observed at the apex and the base. E-cells lack of lipid or glycogen reserves. They can be observed at different mitotic stages.

FIGURE 2. E cells: prominent nucleus and scarce organelles. N: nucleus, R: R cell. MET. X 8,000
F-cells are located at medial and proximal zones of the tubules among R and B-cells. They are cylindrical or dome-like bodies with a centrally or basally located nucleus and apical brush border. Their mitochondria are uniformly distributed around the nucleus, and the Golgi bodies have heavily dilated cisternae. The most conspicuous features of these cells are the dilated Golgi cisternae, and the great development of the RER that surrounds the nucleus and occupies most of the cytoplasm (Fig. 3).
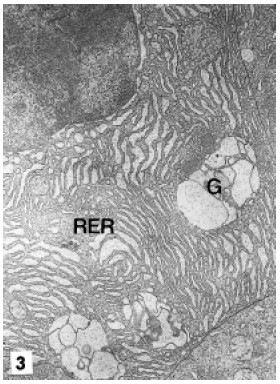
FIGURE 3. F cell. Note the great development of the RER and dilated cisternae of Golgi. G: Golgi, RER: rough endoplasmic reticulum. MET. X 10,000
R-cells are most abundant, and are located at medial and proximal zones of the tubules. They present a polar organization of organelles in three areas: the apical, with a brush border and numerous mitochondria, sER tubules and little vacuoles (Fig. 4); the medial, with the nucleus surrounded by the RER, and the basal, dominated by big mitochondria and a sER-like tubular system (Fig. 5). Glycogen was not observed; however several cells presented lipid droplets of heterogenous sizes and homogenous contents (Fig. 6). R-cells exhibit small primary lysosomes, autophagosomes of different sizes and electrodense residual bodies (Fig. 7).

FIGURE 4. R cell apical area. Observe the conspicuous brush border, endocytotic vesicles (arrows) and the mitochondria concentration. m: mitochondria. MET. X 8,000

FIGURE 5. R cell infranuclear area with RER, large mitochondria and a branched system of sER like tubules. m: mitochondria, RER: rough endoplasmic reticulum, st: sER like tubules. MET. X 12,000
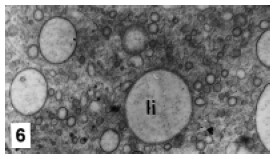
FIGURE 6. Details of lipid droplets in the R cell cytoplasm. li: lipid droplets. MET. X 15,600

FIGURE 7. R cell cytoplasm. Little lysosomes, autophagosomes of different sizes and electrodense residual
bodies. au: autophagosome, r: residual bodies. MET. X 12,000
B-cells are more frequent at the proximal zone of the tubules than at the other zones. They were observed at different stages of their life cycle. At the beginning of the differentiation, they evidence an apical complex which comprises numerous endocytotic channels and vesicles. Endocytotic channels invaginate from the brush border deep into the cell and give rise to endocytotic vesicles. The endocytotic vesicles fuse with Golgi vesicles originating subapical vacuoles (Fig. 8). The nucleus, surrounded by the RER, elongated Golgi cisternae, small lysosomes and autophagosomes can be observed at the medial area of the cell (Fig. 9). At an early maturation stage the apical complex become more important: the apical vacuoles are enlarged by the incorporation of endocytotic vesicles and Golgi vesicles. The coalescence of vacuoles originates a large supranuclear vacuole (Fig. 9), which increases in size by the incorporation of new vacuoles (Fig. 10). In the mature cell the nucleus and the rest of the organelles are displaced towards the cell base by the expansion of the supranuclear vacuole. The completely mature cell is like a blister, the large vacuole occupies nearly the whole cytoplasm (Fig. 11) and the brush border is reduced in length. At the end of the maturation period, the large vacuole is eliminated by holocrine secretion. The complete cell protrudes from the epithelium and loses contact with the basal lamina.
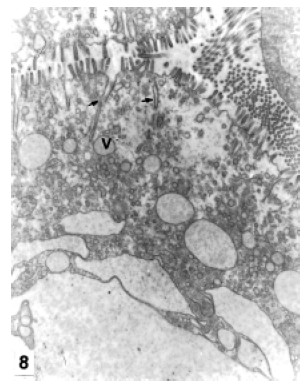
FIGURE 8. B cell. Apical complex at the beginning of the differentiation. Endocytotic channels (arrows), v: endocytotic vesicles. MET. X 8,000
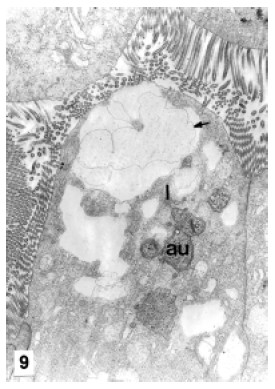
FIGURE 9. B cell apex. Note the endocytotic vesicles fusion originating a big supranuclear vacuole (arrow). au: autophagosome, l: lysosome. MET. X 8,000

FIGURE 10. B cell. Detail of supranuclear vacuole enlargement by coalescence of new vesicles. MET. X 8,000

FIGURE 11. Mature B cell. The central vacuole occupies nearly the whole cytoplasm. V: vacuole. X 13,700
Discussion
The general epithelial cytology of the hepatopancreas in P. argentinus is consistent with that observed in other decapods (Al-Mohanna et al., 1985; Icely and Nott, 1992; Johnston et al., 1998).
E-cells are undifferentiated, with embryonic ultrastructural features, and are the only cells showing mitotic activity. These findings confirm the previous observations by Sousa and Petriella (2000) in the same species and are coincident with those observed in other decapod species (Travis, 1955; Al-Mohanna and Nott, 1989; Petriella and Fonalleras, 1997).
F-cells are located among R and B cells at the medial zone of the tubules and have basophilic cytoplasm because of the high RER content (Vogt et al., 1989; Al-Mohanna and Nott, 1989; Sousa and Petriella, 2000). Ultrastructurally, they are characterized by a considerable amount of RER and prominent Golgi bodies. Both characteristics evidence an active protein synthesis. These cells are considered as the only site of digestive enzyme synthesis (Icely and Nott, 1992). Different enzymes were localized by immunohistochemistry and immunofluorescence, such as trypsine, a-amylase, astacin, and chimotrypsin, in the F-cells of various decapods (Malcoste et al., 1983; Vogt et al., 1989; Johnston et al., 1998). In the present study, no reserves were found in this cell type; apparently the main function of these cells is the synthesis and secretion of proteins, presumably digestive enzymes.
The absorptive function of P. argentinus R cells is supported by the presence of lipid droplets in the cytoplasm, the conspicuous brush border and the apical tubular system. The apical sER system in association with the apical mitochodria is also observed in other decapods and is involved in nutrient absorption (Vogt, 1994; Johnston et al., 1998). The nutrient reserves, stored during intermoult, are mobilized to provide energy during starvation periods (late premoult) (Vogt, 1996; Sousa and Petriella, 2001). Glycogen was not found in R cells, coincidentally a little amount of this nutrient was found in a previous study during intermoult in the same species (Sousa and Petriella, 2001). These prawns feed actively during intermoult so it is possible that the storage of glycogen is not necessary because of the constant supply (Chang and O'Connor, 1983; Sousa and Petriella, 2001). The basal tubule system of sER found in R cells of P. argentinus closely related to mitochondria seems to be involved in the delivery of nutrients to other organs via the hemolymph (Vogt, 1985; 1994; Johnston et al., 1998). Autophagosomes and residual bodies were present in various R cells of P. argentinus; supranuclear autophagosomes are interpreted as sites of intracellular waste deposition (Vogt, 1994). Residual bodies observed in P. argentinus R cells are consistent with the metal storing vacuoles and residual bodies present in Thenus orientalis and Penaeus semisulcatus, respectively (Al- Mohana and Nott, 1987; 1989; Johnston et al., 1998). These cells detoxify heavy metals by their accumulation in a soluble form in the cytoplasm before excretion (Vogt, 1987; Johnston et al., 1998).
B-cells of P. argentinus, like those in other decapods (Icely and Nott, 1992; Al-Mohana and Nott, 1989; Johnston et al., 1998; Vogt, 1993) are characterized by an endocytotic apical complex and a central vacuole; these ultrastructural features indicate an absorbing and degrading function (Hopkin and Nott, 1980; Vogt, 1993; 1994). The gradual enlargement of the central vacuole in P. argentinus indicates the absorption of materials from the tubular lumen, but no nutrients were detected in B-cells cytoplasm. Vogt (1994) proposed that B cells degrade exhausted digestive enzymes and waste products which remain in the tubules after absorption of nutrients.
In summary, the ultrastructure of F-cells in P. argentinus, like in most of decapods, is consistent with an active synthesis and secretion of proteins. R-cells are implicated in absorption and storage of nutrients which are exported to other organs through the basal sER-like tubule system. B cells ultrastructural features indicate an absorptive and degrading function; mature B cells, with the central vacuole replete of wastes, are discharged by holocrine secretion to the tubular lumen. The present fine analysis supports a previous conclusion about the origin of F, R and B cells (Sousa and Petriella, 2000), which considers that these specialized cells originate independently from embryonic cells.
Acknowledgments
This research was supported by a grant from Universidad Nacional de Mar del Plata (15 E 136).
References
1. Al-Mohanna SY, Nott JA (1987). R-cells and the digestive cycle in Penaeus semisulcatus (Crustacea: Decapoda). Mar Biol 95: 129-137. [ Links ]
2. Al-Mohanna SY, Nott JA (1989). Functional cytology of the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda) during the moult cycle. Mar Biol 101: 535-544. [ Links ]
3. Al-Mohanna SY, Nott JA, Lane JW (1985). Mitotic E- and secretory F-cells in the hepatopancreas of the shrimp Penaeus semisulcatus (Crustacea: Decapoda). J Mar Biol Ass UK 65: 901-910. [ Links ]
4. Boschi EE (1981). Decapoda Natantia. In: Fauna de agua dulce de la República Argentina. Vol. 26. FECIC, Buenos Aires, pp. 61. [ Links ]
5. Collins PA (1999). Feeding of Palaemonetes argentinus (Decapoda: Palaemonidae) from an oxbow lake of the Paraná River, Argentina. J Crust Biol 19(3): 485-492. [ Links ]
6. Chang ES, O'Connor JD (1983). Metabolism and transport of carbohydrates and lipids. In: The Biology of Crustacea. Vol. 5. D.E. Bliss, Ed. Academic Press, New York, pp. 263-287. [ Links ]
7. Díaz AC, Petriella AM, Sousa LG (1998). Setogenesis and growth of the freshwater prawn Palaemonetes argentinus (Decapoda, Caridea, Palaemonidae). Iheringia Sér Zool 85: 59-65. [ Links ]
8. Gibson O, Barker PL (1979). The decapod hepatopancreas. Oceanogr. Mar Biol Annual Rev 17: 285-346. [ Links ]
9. González Sagrario MA, Aizpún de Moreno JE, Moreno VJ, Escalante AH (1998). Dynamics of organochlorine compounds in different trophic levels of Los Padres Pond in Argentina. I. Pesticides. Environ Sci 6(3): 153-170. [ Links ]
10. Hopkin SP, Nott JA (1980). Studies on the digestive cycle of the shore crab Carcinus maenas (L) with special reference to the B-cells in the hepatopancreas. J mar biol Ass UK 60: 891- 907. [ Links ]
11. Icely JD, Nott JA (1992). Digestion and absorption: digestive system and associated organs. In: Microscopic anatomy of invertebrates: Decapod, Crustacea. Vol. 10. F.W. Harrison and A.G. Humes, Eds. Wily-Liss Inc., N.Y., pp 147-201. [ Links ]
12. Johnston DJ, Alexander CG, Yellowhees D (1998). Epithelial cytology and function in the digestive gland of Thenus orientalis (Decapoda, Scyllaridae). J Crust Biol 18(12): 271-278. [ Links ]
13. Loizzi RF (1971). Interpretation of crayfish hepatopancreatic function on fine structural analysis of epithelia cell lines and muscle network. Z Zellforsch 113: 420-440. [ Links ]
14. Malcoste R, Van Wormhoudt A, Bellon-Humbert C (1983). Preliminary results on the characteristics of the hepatopancreas of the prawn Palaemon serratus Pennant (Crustacea, Decapoda, Natantia) cultivated in vitro. Comptes Rendus Hebdomadaires des Séances de L'Académie des Sciences, Paris 295 Sér 3: 597-602. [ Links ]
15. Miglioranza KM, Menone M, Gonzalez M, Gerpe M, Aizpún J, Moreno J (2003). Fuentes terrestres de contaminación marina: plaguicidas organoclorados en sedimentos superficiales de arroyos de la provincia de Buenos Aires. V Jornadas de Ciencias del Mar, XIII Coloquio Argentino de Oceanografía. Mar del Plata, Argentina, pp. 140. [ Links ]
16. Morrone JJ, Lopreto EC (1995). Parsimony analysis of endemicity of freshwater Decapoda (Crustacea: Malacostraca) from Southern South America. Neotropica 41: 3-8. [ Links ]
17. Petriella AM, Fonalleras MC (1997). Citoarquitectura del hepatopáncreas del camarón Artemesia longinaris Bate (Crustacea, Decapoda, Penaeidae). Physis Secc A 55(128-129): 25-30. [ Links ]
18. Popescu-Marinescu V, Manolache V, Nastasescu M, Marinescu C (1997). Structural modifications induced by cooper in Astacus leptodactylus (Crustacea, Decapoda) hepatopancreas. Rom J Biol Sci 1 (2), 99-105. [ Links ]
19. Sousa LG, Petriella AM (2000). Histology of the hepatopancreas of the freshwater prawn Palaemonetes argentinus (Crustacea, Caridea). Biocell 24(3): 189-195. [ Links ]
20. Sousa LG, Petriella AM (2001). Changes in the hepatopancreas histology of Palaemonetes argentinus (Crustacea, Caridea) during moult. Biocell 25(3): 275-281. [ Links ]
21. Spivak ED (1997). Life history of a brackish-water population of Palaemonetes argentinus (Decapoda: Caridea) in Argentina. Ann Limnol 33: 179-190. [ Links ]
22. Storch V, Welsch U (1977). Elektronenmikroskopische und enzymhistochemische Untersuchungen der Mitteldarmdrüse der landlebenden Decapoden Coenobita rugosus und Ocypode ceratophthalma. Zool Jb (Anat Ontogenie Tiere) 79: 25-39. [ Links ]
23. Travis DF (1955). The moulting cycle of the spiny lobster Panulirus argus Latreille. II. Pre-ecdycsial histological and histochemical changes in the hepatopancreas and integumental tissues. Biol Bull 108: 88-112. [ Links ]
24. Vogt G (1985) Histologie und cytologie der mitteldardrüse von Penaeus monodon (Decapoda). Zool Anz 215: 61-80. [ Links ]
25. Vogt G (1987). Monitoring of environmental pollutants such as pesticides in prawn aquaculture by histological diagnosis. Aquaculture 67: 157-164. [ Links ]
26. Vogt G (1993). Differentiation of B-cells in the hepatopancreas of the prawn Penaeus monodon. Acta Zool 74: 51-60. [ Links ]
27. Vogt G (1994). Life-cycle and functional cytology of the hepatopancreas cells of Astacus astacus (Crustacea, Decapoda). Zoomorphology 114: 83-101. [ Links ]
28. Vogt G (1996). Morphology and physiology of digestive epithelia in decapod crustaceans. Pflügers Archiv European J Physiol 431, supplement 2: 239-240. [ Links ]
29. Vogt G, Stöcker W, Storch V, Zwilling R (1989). Biosynthesis of Astacus protease, a digestive enzyme from crayfish. Histochemistry 91: 373-381. [ Links ]
Received on Juny 8, 2004.
Accepted on November 3, 2004.














