Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.29 n.2 Mendoza abr./ago. 2005
Structure and secretory activity of cultured chondrocytes from patients with osteoarthritis
Hilda L. Montrull, Nilda Y. Brizuela, Silvia L. Demurtas, Luis Spitale* and Carlos I. Meirovich
Departamento de Farmacología, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Argentina.
* II Cátedra de Anatomía y Fisiología Patológica, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Argentina.
Address correspondence to: Hilda L. Montrull. Alvear 173, 1º Piso, Dpto. "A", CP 5000, Córdoba, ARGENTINA. FAX: (+54-351) 4229902. E-mail: rcalberd@nt.com.ar
Abstract: Cartilage samples were taken from OA patients in order to describe and quantify pro-inflammatory mediators. Samples were cultured under aseptic conditions in Dulbecco's modified Eagle medium at 37°C for 10 days. Control samples, taken from non-inflammatory cartilage, were cultured under the same conditions. The levels of NO-2 and NO-3 were measured in the supernatant using a spectrophotometric assay. The activity of MMP-1 was quantified by ELISA.
The concentration of NO-x was 47.3 ± 4.1 µM in the OA cartilague and 10.7 ± 1.8 µM in the controls. The average MMP-1 activity was 3,650 ± 387 ng/ml in the OA cartilage and 2,150 ± 190 ng/ml in the control samples. These increased values of MMP-1 and NO-x observed in the OA cartilage suggest a higher catabolic activity.
A morphological analysis of OA chondral tissue using light microscopy shows that the surface of the tissue is characterized by the presence of aggregated chondrocytes or "clones" but in the deeper areas isolated cells are found.
These results could be a significant contribution towards the identification of biological markers indicating the presence of OA activity.
Keywords: Osteoarthritis. Chondrocytes. Collagenase.
Introduction
Osteoarthritis (OA) is a chronic, degenerative disease of the articular cartilage. It is the result of a chain of events that involves mechanical and biological processes of the muscle-bone system that destabilize the equilibrium of chondral synthesis and degradation.
Growth factors and cytokines generated in the synovial membrane and which diffuse throughout the cartilage via the synovial fluid (Pelletier et al., 1993), have an important role in the characteristic alterations caused by OA. Among these factors, the alpha -tumoral necrosis factor (TNF-α) and the beta-interleukin IL-1 are found unchain the inflammatory process (Martel- Pelletier et al., 1999; Fernandes et al., 2002). Other important factors involved are IL-8, a potent chemotaxic for polimorphonuclear neutrophils (PMN), the leukemia inhibitory factor (LIF), IL-6, IL-11 and IL-7, all of which are responsible for the "induction" of metalloproteases (collagen, stromelysin) and for the increase in nitric oxide (NO) production. The increase of this last catabolic factor is due to chondrocyte-specific inducible NO synthase (iNOS) and causes the increase of metalloproteinases and the decrease of glycosaminoglicans (GAGs), thus augmenting the catabolism of OA articular cartilage. (Amin and Abramson, 1998). Both pro-inflammatory cytokines and metalloproteinases not only stimulate their own production but also the activity of osteoclasts (Abramson et al., 2000; Martel-Pelletier et al., 1994; Chevalier, 1997; Tetlow et al., 2001).
Native articular cartilage is organized in distinct areas that are clearly evidenced because they are formed by cells of different morphological and mechanical properties. This particular organization is important for the articular cartilage to have a normal physiological behaviour. Chondrocytes are responsible for developing and maintaining the cartilage structure in areas parallel to the articular surface. In OA cartilage these properties are altered.
It is still necessary to study different aspects of the pathogeny of OA in order to be able to fully understand the natural development of this disease. Therefore, the aim of this study was to describe histopathological changes and quantify some pro-inflammatory factors in patients suffering from OA.
Material and Methods
Cartilage samples
Cartilage samples were obtained when joint replacement surgery was being undertaken in patients with OA, who fulfilled the American College of Rheumatology criteria for this disease (Altman et al., 1986). These experiments were performed in accordance with a protocol approved by the Committee of Ethics of the Hospital de Clínicas. A written consent was signed by all patients authorising the use of their tissues for this research. Cartilage samples taken from a group of 8 surgical patients with traumatic articular injury were used as controls. The size of the samples extracted was of approximately 5 x 3 mm.
Cartilage culture
Cartilage samples were washed in calcium- and magnesium-free phosphate buffered saline (DPBS) and finely ground. Chondrocytes were obtained by digesting the articular cartilage with 0.2% pronase (Sigma, St. Louis, MO, USA) for 1 h, followed by digestion with 0.2% clostridia collagenase (Sigma) for 3 h at 37°C in high-glucose Dulbecco's modified Eagle medium (DMEM; Life Technologies, Rockville, MD, USA) containing an antibiotic-antimycotic solution (100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B; Life Technologies). After removing undigested cartilage with a 70 µg nylon mesh (Cell Strainer; Falcon, Franklin Lakes, NJ, USA), the chondrocytes were collected by centrifugation, washed twice and then resuspended in the DMEM medium with 10% fetal bovine serum (FBS; Life Technologies). Finally, they were plated in 100-mm tissue culture dishes for expansion at 37°C in a 5% CO2 humidified atmosphere for 10 days (Schell-Lab Oregon USA). Every 2 days the medium was removed and stored at -20ºC until the corresponding chemical determinations were undertaken.
NO-x assay
NO release was estimated from the amounts of nitrite (NO-2) and nitrate (NO-3) present in the incubation medium. NO3- was calculated by first reducing it to NO-2 in the presence of Cd (Cortas and Wakid, 1990) and then NO-2 was determined by a colorimetric assay based on the Griess reaction (Green, 1982) (Metrolab 1600, Argentina).
Metalloproteinase-1 assay
The concentration of MMP-1 in the supernatant of the cell cultures was determined using specific ELISA (Amersham Biosciences).
Protein assay
Proteins were quantified using bicinchonic acid (Hill and Straka, 1988).
Anatomopathological analysis
For the anatomo-pathological analysis cultured cartilage fragments were placed in 5% formaldehyde. Samples were dehydrated with increasing concentrations of alcohol and finally embedded in paraffin. Sections were then stained with hematoxylin-eosin (H/E).
Statistical analysis
The results are expressed as the mean ± standard error. Data were analysed with the unpaired t-test. A pvalue <0.05 was considered significant.
Results
1. NO-x
Figure 1 shows the total concentrations of NO during the 10 days of culture (pooled data from days 2, 4, 6, 8 and 10). The mean concentration of NO-2 plus NO-3 (NO-x) from samples of patients with OA (47.3 ± 4.1 µM) was significantly different (p<0.001) from the control samples (10.7 ± 1.8 µM).

FIGURE 1. NO-x . The levels of stable metabolites of nitric oxide (NO) were measured in culture supernatants using the Griess reaction, and are expressed as µM. Bars represent the mean ± SEM. OA: osteoarthritic sample; C: control sample. * indicates significant difference, p<0.001, n=8.
2. Collagenase concentration
Figure 2 shows the total concentrations of MMP-1 during the 10 days of culture (pooled data from days 2, 4, 6, 8 and 10). The concentration of MMP-1 in OA cartilages was of 3,650 ± 387 ng/ml, significantly higher (p<0.001) than the concentration found in the control samples (2,150 ± 190 ng/ml).

FIGURE 2. Collagenase production. The activity of MMP-1 was determined by ELISA and is expressed as ng/ml. Bars represent the mean ± SEM. OA: osteoarthritic sample; C: control sample. * indicates significant difference, p<0.001, n=8.
3. Morphological characteristics
Histological evaluation of control cartilage slices confirmed that samples had been taken from the upper, middle, and lower areas of the cartilage block. The upper area had the highest cellular content, followed by the middle area and then the lower area. Cells of the upper area were smaller than those of the middle and lower areas. The cells of the articular surface of the upper area were either flattened or ellipsoidal and were parallel to the surface. The cells in the lower area were larger in volume and were arranged in groups of 2-8 cells (clones) (Fig. 3). These "clones" of aggregated chondrocytes are typical of OA and are more frequently found in the upper superficial layers of the culture (Fig. 4). They have characteristic large euchromatic nuclei and appear in lacuna areas. However, neither the mechanism nor the sequence of their formation has yet been elucidated.
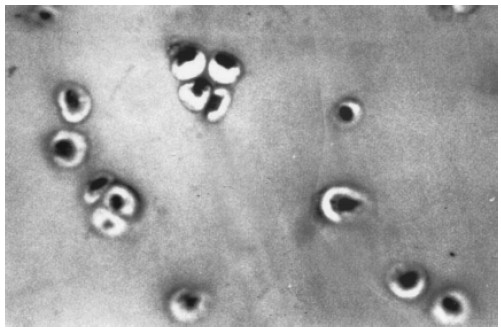
FIGURE 3. Light microscopy micrograph of chondrocyte culture. Chondrocytes are found in regular groups with no modifications in their nuclear and cytoplasmic characteristics. Hematoxyline-eosin staining. (X 400)

FIGURE 4. Light micrograph of chondrocyte culture from osteoarthritic patients. Chondrocyte aggregates or "clones" are found in a great matrix lacune, on the surface layers. Hematoxylin-eosin staining. (X 400)
Discussion
Our laboratory has performed previous studies using chondrocytes from patients without an inflammatory pathology. We have observed that the addition of IL-1 "in vitro" causes significant changes in the generation of adhesion molecules, particularly in enzymes such as metalloproteases and in pro-inflammatory factors such as NO, thus verifying that interleukines are important in the changes that take place in chondral cultures ( Brizuela et al., 2000).
In the present work we have been able to observe that cultures of chondrocytes extracted from OA patients are characterised by the presence of cell populations with morphological differences. In accordance to the classification proposed by Kouri et al. (1996) for different types of chondrocytes, in OA cultures we have been able to describe the presence of "clones" in both the surface and the intermediate area, that would be responsible for the catabolic mechanisms characteristic of this disease. We have previously determined that in osteoarthritic chondrocytes the production of catabolic enzymes (collagenase and metalloproteinases) is increased by 80% compared to controls (Brizuela et al., 2000).
The analysis of NO metabolites shows that the NO-x concentration of OA cultures is five times higher than the levels found in the control samples. This is in accordance with the increased number of proteolytic enzymes causing great cartilage damage and the lower level of reparation in OA tissues, both characteristic consequences of the chronic inflammatory process present in this illness.
In the present investigation we have observed that chondrocyte cultures of patients with OA are characterised by the presence of cell populations with morphological differences. Coinciding with the classification proposed by Kouri and col. for the different types of chondrocytes found in different cultures, in OA cultures we observed "clones" on both the surface and the middle areas of the culture. We have previously demonstrated that the production of catabolic enzymes (collagenase or metalloproteinase-1) in osteoarthritic chondrocytes is increased by 80% compared to the control cultures (Brizuela et al., 2000). It is possible that these "clones" are the areas responsible for the catabolic mechanisms characteristic of this disease.
Conclusions
In the present study we found that, in cultures of human chondral OA cartilage, the presence of aggregated cellular groups (clones) on the surface and isolated secretory cells in the deeper layers were characteristic. The production of metallopreoteases was very high compared to the control samples and the increased NO production suggests that the tissue degradation tends to exceed the reparative function. Finally, the results also confirm that cytokines, proteolytic enzymes and autacoids have different and determined roles. It is possible to infer that new insights towards the knowledge of OA physiopathology have been achieved.
Acknowledgements
We are grateful to the Department of Pharmacology of the Universidad Complutense de Madrid, in particular to the Director Dr. Pedro Lorenzo Fernandez, for his technical assistance, methodological suggestions and his moral and human support. This work was partially supported by the Secretaría de Ciencia y Tecnología (SECyT) of the Universidad Nacional de Córdoba.
References
1. Abramson SB, Attur M, Amin AR, Clancy R (2000). Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr Rheumatol 2(6): 447-453. [ Links ]
2. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, Mankin H, McShane DJ, Medsger T, Meenan R Jr, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F (1986). Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29: 1039-1049. [ Links ]
3. Amin AR, Abramson SB (1998). The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol 10: 263-268. [ Links ]
4. Brizuela NY, Demurtas SL, Montrull HL (2000). Markers of inflammatory activity in chondrocyte culture. Biocell 24: 62. [ Links ]
5. Chevalier X (1997). Up regulation of enzymatic activity by interleukin-1 in osteoarthritis. Biomed Pharmacother. 51: 58- 62. [ Links ]
6. Cortas NK, Wakid NW (1990). Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem, 36: 1440-1443. [ Links ]
7. Fernandes JC, Martel-Pelletier J, Pelletier JP (2002). The role of cytokines in osteoarthritis pathophysiology. Biorheology 39: 237-246. [ Links ]
8. Green LC (1982). Analysis of nitrate, nitrite, and [15-N] nitrate in biological fluid. Annal Biochem 126: 131-138. [ Links ]
9. Hill HD, Straka JG (1988). Protein determination using bicinchonic acid in the presence of sulfhydryl reagents. Annal Biochem. 170: 203-208. [ Links ]
10. Kouri JB, Arguello C, Quinteros M, Chico A, Ramos MA (1996). Variability in the cell phenotype of aggregates or "clones" of human ostearthritic cartilage. A case report. Biocell 20: 191- 200. [ Links ]
11. Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP (1994). Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest 70: 807-815. [ Links ]
12. Martel-Pelletier J, Alleddine N, Pelletier JP (1999). Cytokines and their role in the pathophysiology of osteoarthritis. Frontiers in Bioscience. 4: 694-703. [ Links ]
13. Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel- Pelletier J (1993). Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 19: 545-568. [ Links ]
14. Tetlow LC, Adlam DJ, Woolley DE (2001). Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 44: 585-594. [ Links ]
Received on March 19, 2004.
Accepted on February 22, 2005.














