Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.29 no.2 Mendoza Apr./Aug. 2005
Reactive oxygen species in bovine embryo in vitro production
G.C. Dalvit, P.D. Cetica, L.N. Pintos and M.T. Beconi
Area of Biochemistry, School of Veterinary Sciences, University of Buenos Aires. Chorroarín 280, (C1427CWO) Buenos Aires, Argentina.
Address correspondence to: Martha T. Beconi, PhD. Area de Química Biológica, Facultad de Ciencias Veterinarias, Universidad de Buenos Aires, Chorroarín 280, C1427CWO Buenos Aires, ARGENTINA. Phone/FAX: (+54-11) 5248452. E-mail: beconi@fvet.uba.ar
Abstract: Oxidative modifications of cell components due to the action of reactive oxygen species (ROS) is one of the most potentially damaging processes for proper cell function. However, in the last few years it has been observed that ROS participate in physiological processes. The aim of this work was to determine ROS generation during in vitro production of bovine embryos. Cumulus-oocyte complexes were recovered by aspiration of antral follicles from ovaries obtained from slaughtered cows and cultured in medium 199 for 22 h at 39ºC in 5% CO2: 95% humidified air. In vitro fertilization was carried out in IVF-mSOF with frozenthawed semen in the same culture conditions and embryo in vitro culture in IVC-mSOF at 90% N2: 5% CO2: 5% O2. ROS was determined in denuded oocytes and embryos at successive stages of development by the 2´,7´-dichlorodihydrofluorescein diacetate fluorescent assay. ROS production was not modified during oocyte maturation. However, a gradual increase in ROS production was observed up to the late morula stage during embryo in vitro culture (P<0.05). In expanded blastocysts, ROS level decreased to reach values similar to the corresponding in oocytes. In the bovine species, the variation in ROS level during the complete process of embryo in vitro production was determined for the first time.
Keywords: Reactive oxygen species. Oxidative stress. Oocyte. Embryo. Bovine
Introduction
The concept of oxidative stress has been widely used in biological sciences to describe an enhanced state of oxidants or a lack of antioxidants in cells, a situation in which the concentration of reactive oxygen species (ROS) increases above its biological values (Gonzales Flecha et al., 1993; Sikka, 2001). The oxidative modification of cell components due to the action of ROS is one of the most potentially damaging processes for proper cell function that may lead to cell death by necrosis or apoptosis (Sarafian and Bredesen,1994; Yang et al., 1998).
Embryo in vitro culture affords higher oxygen concentrations than in vivo environments, resulting in increased ROS production (Luvoni et al., 1996). In the bovine species, the procedure for producing embryos in vitro is still unsatisfactory, ranging 30-40% of blastocyst rate at day seven. ROS generation has been implicated as a major cause of low percentages of bovine embryo in vitro production. ROS has been suggested to participate in meiotic arrest in oocytes (Downs and Mastropolo,1994; Nakamura et al., 2002), embryonic block and cell death (Hashimoto et al., 2000). However, during the last years it has been observed that physiological concentrations of ROS participate in normal cell processes as major factors in growth and development regulation (Hancock et al., 2001).
Therefore, in the bovine species the role of ROS as regards in vitro maturation, fertilization and embryo development, remains controversial. Although the effect of antioxidants in these processes has been described, there are no studies that document whether during in vitro maturation and embryo development conditions there are variations in ROS production attributable to oocytes and embryos. Accordingly, the aim of this work was to determine the production of ROS in one of the most widely systems used for bovine in vitro production.
Materials and Methods
Ovaries were obtained from slaughtered cows. Cumulus- oocyte complexes (COCs) were recovered by aspiration of antral follicles and only good quality oocytes completely surrounded by compact and thick cumulus were used. COCs were matured in medium 199 supplemented with 10% steer serum and 50 mg/l gentamicine sulfate at 39ºC during 22 h in 5% CO2 with humidified air. Meiotic maturation was evaluated using 40% of the samples as described Cetica et al. (1999).
In vitro fertilization was carried out using frozen semen, thawed at 37ºC in mSOF medium (Takahashi and First,1992) with 10 mmol/l theophylline, centrifuged at 500g twice for 5 min and resuspended in the fertilization medium to a final concentration of 2x106 motile spermatozoa/ml. Fertilization was carried out in IVFmSOF medium (mSOF supplemented with 10 iu/ml heparin and 5 mg/ml BSA) under mineral oil at 39ºC, 5% CO2 and 100% humidity during 20 h. Zygotes were denuded by pipetting and placed in IVC-mSOF (mSOF supplemented with 30 µl/ml of MEM solution of amino acids, 10 µl/ml of MEM solution of non-essential amino acids, 2 mmol/l L-glutamine, 6 mg/ml BSA and 5% FBS) under mineral oil at 90% N2: 5% CO2: 5% O2 and 100% humidity during 24 h. In vitro fertilization percentage was determined by evaluating the number of embryos divided into 2 or more blastomeres.
Embryo in vitro culture was carried out in IVCmSOF medium under mineral oil at 90% N2: 5% CO2: 5% O2 and 100% humidity. Blastocyst percentage was determined at the seventh day of insemination. Only grade 1 (excellent) and 2 (good) embryos at the expected stage of development for each age were used (Kennedy et al., 1983).
To measure the production of ROS, immature and matured oocytes were completely denuded by vortex agitation in physiological saline solution for 1 min and then washed to eliminate cumulus cells. Samples of 20 denuded oocytes or embryos at different stages of development were incubated in 40 mmol/l Tris-HCl buffer pH 7.0 at 37°C for 30 min in the presence of 5 µmol/l 2´,7´-dichlorodihydrofluorescein diacetate (DCHFDA), washed, sonicated at 50 W for 1 min and centrifuged at 4ºC and 10,000g for 20 min. Fluorescence was monitored in the supernatant using a spectrofluorometer at 488-nm excitation and at 525-nm emission (LeBel et al., 1992; Cetica et al., 2001). Corrections for autofluorescence were made by inclusion of parallel blanks in each experiment.
Data were expressed as Arbitrary ROS Units/denuded oocyte or embryo/min (means ± SEM) x 10-3 of seven replicates for each cellular stage. Statistical comparisons were made by ANOVA.
Results
In our system of bovine embryo in vitro production, the percentages of meiotic maturation, in vitro fertilization and blastocysts obtained at day 7 were 85%, 77% and 38%, respectively.
ROS determination by spectrofluorometry using DCHFDA showed that their generation remained constant during bovine oocyte in vitro maturation due to ROS level was not significantly different between immature and matured denuded oocytes (Fig. 1).
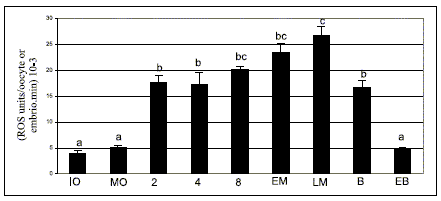
FIGURE 1. ROS levels determined by spectrofluorometry using 2´,7´- dichlorodihydrofluorescein diacetate assay during bovine oocyte maturation and embryo development in vitro.
IO, immature oocyte; MO, matured oocyte; 2, 2 cell embryo; 4, 4 cell embryo; 8, 8 cell embryo; EM, early morula; LM, late morula; B, blastocyst; EB, expanded blastocyst. Values are expressed as (means ± SEM) 10-3 of 7 replicates for each stage. a, b, c bars with different superscripts are significantly different (P<0.05).
A significant increase in ROS level in 2 cell embryo was detected with respect to the oocyte (P<0,05) and then, during embryo in vitro culture there was a gradual enhancement starting from the 2-cell embryo up to the late morula stage (P<0.05) (Fig. 1).
In the blastocyst, ROS values decreased until they reached levels similar to those of oocytes in the expanded blastocyst stage (P<0.05) (Fig. 1).
Discussion
It has been shown that DCFHDA probe is oxidized by hydrogen peroxide, its derived oxidants, other peroxides and indirectly by the superoxide anion when generating hydrogen peroxide, thus providing an useful test to evaluate ROS production (LeBel et al., 1992).
The production of ROS in denuded bovine oocytes from immature and in vitro matured COCs was unaltered by maturation, indicating that culture conditions employed were not responsible for oxidative stress in the female gamete. The maintenance of ROS production levels during maturation could be due partly to the action of the antioxidant system described in bovine COCs (Cetica et al., 2001; Dalvit et al., 2005). This steady state concentration of ROS could be necessary for the maturation process, as suggested by Blondin et al. (1997), who documented that a certain production of ROS during bovine oocyte in vitro maturation is required to increase blastocyst percentage.
The significant enhace in ROS generation observed in 2 cell embryo with respect to the oocyte would be related to diverse factors. It could be due to modifications in embryo in vitro culture conditions with regard to those of maturation, including changes in culture medium, the corresponding gas mixture used and the total separation of cumulus cells starting from the zygote stage, or the increase of metabolic activity due to embryo cleavage.
The gradual increase in ROS levels from the 2-cell embryo up to the late morula stage could depend on the metabolic change undergone by the embryo during its development. Thompson et al. (1996a) reported a sustained increase in oxygen, glucose and pyruvate uptake during embryo in vitro development. This enhancement in oxidative metabolism of the embryo could be linked to the detected increase in ROS level.
The decrease of ROS values detected in blastocysts may be related to the onset of cellular differentiation. This onset would modify oxygen metabolism and/or the acquisition of autonomy by the embryonic genome to induce the synthesis of the antioxidant system that controls ROS production. It has been demonstrated that oxygen uptake diminishes in expanded blastocysts due to they have lower physiological ATP demand (Thompson et al., 1996b) because glycolysis begins to contribute to ATP production (Thompson et al., 1996a, 2000). Alternatively, the embryonic genome may acquire the autonomy to induce its own antioxidant system to control ROS production. Harvey et al. (1995) reported that in the bovine species certain antioxidant enzyme transcripts increase or appear in the blastocyst stage.
In conclusion, the present work is the first in determining the level of ROS production during the entire process of bovine embryo in vitro production. On the basis of our results, it is suggested that oocyte maturation conditions are not responsible for oxidative stress. However, metabolic changes during embryo development would lead to a gradual increase in ROS production, to peak in the late morula stage, then dropping abruptly in the expanded blastocyst stage. Given such data, in future work it would be of interest to control ROS levels by focusing on the successive stages of embryo in vitro culture and to determine whether they lead to cell deterioration due to oxidative stress or whether they are involved in the normal process of embryo development.
References
1. Blondin P, Coenen K, Sirard MA (1997). The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl 18: 454-460. [ Links ]
2. Cetica PD, Pintos LN, Dalvit GC, Beconi MT (2001). Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 51: 57-64. [ Links ]
3. Cetica PD, Dalvit GC, Beconi MT (1999). Study of evaluation criteria used for in vitro bovine oocyte selection and maturation. Biocell 23: 125-133. [ Links ]
4. Dalvit G, Llanes SP, Descalzo A, Insani M, Beconi M, Cetica P (2005). Effect of alpha-tocopheron and ascorbic acid in bovine oocyte in vitro maturation. Reprod Dom Anim 40: 93-97. [ Links ]
5. Downs S, Mastropolo A (1994). The participation of energy substrates in the control of meiotic maturation in murine oocytes. Dev Biol 162: 154-168. [ Links ]
6. Gonzales Flecha B, Reides C, Cutrin JC, Lluesuy S, Boveris A (1993). Oxidative stress produced by suprahepatic occlusion and reperfusion. Hepatol 18: 881-889. [ Links ]
7. Hancock JT, Desikan R, Neill SJ (2001). Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 29: 345-350. [ Links ]
8. Harvey MV, Arcellana-Panlilio MY, Zhang X, Schultz GA, Watson AJ (1995). Expression of genes encoding antioxidant enzymes in preimplantation mouse and cow embryos and primary bovine oviduct cultures employed for embryo coculture. Biol Reprod 53: 532-540. [ Links ]
9. Hashimoto S, Minami N, Yamada M, Imai H (2000). Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev 56: 520-526. [ Links ]
10. Kennedy LG, Boland MP, Gordon I (1983). The effect of embryo quality at freezing on subsecuent development of thawed cow embryos. Theriogenology 19: 823-832. [ Links ]
11. LeBel CP, Ischiropoulos H, Bondy SC (1992). Evaluation of the probe of 2´,7´-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5: 227-231. [ Links ]
12. Luvoni GC, Keskintepe L, Brackett BG (1996). Improvement in bovine embryo production in vitro by glutathione-containing culture media. Mol Reprod Dev 43: 437-443. [ Links ]
13. Nakamura Y, Nagamata Y, Sugino N, Takayama H, Kato H (2002). Nitric oxide inhibits oocyte meiotic maturation. Biol Reprod 67: 1588-1592. [ Links ]
14. Sarafian TA, Bredesen DE (1994). Is apoptosis mediated by reactive oxigen species? Free Radic Res 21: 1-8. [ Links ]
15. Sikka SC (2001). Relative impact of oxidative stress on male reproductive function. Curr Med Chem 8: 851-862. [ Links ]
16. Takahashi Y, First NL (1992). In vitro development of bovine onecell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37: 963-978. [ Links ]
17. Thompson JG, McNaughton C, Gasparinni B, McGowan LT, Tervit HR (2000). Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos. J Reprod Fertil 118: 47-55. [ Links ]
18. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ (1996a). Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fert 106: 299-306. [ Links ]
19. Thompson JG, Partridge RJ, Houghton FD, Kennedy CJ, Pullar D, Leese HJ (1996b). Oxygen consumption by Day 7 bovine blastocysts: determination of ATP production. Anim Reprod Sci 43: 241-247. [ Links ]
20. Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS (1998). Detection of reactive oxygen species (ROS) and apoptosis in human fragmentated embryos. Hum Reprod 13: 998-1002. [ Links ]
Received on April 28, 2004.
Accepted on March 3, 2005.














