Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.30 n.1 Mendoza ene./abr. 2006
Synergism of flavonoids with bacteriostatic action gainst Staphylococcus aureus ATCC 25 923 and Escherichia coli ATCC 25 922
María De Los A. Alvarez, Nora B. Debattista, and Nora B. Pappano
Laboratory of Physical-Chemistry, Department of Chemistry, San Luis National University. San Luis, Argentina.
Address correspondence to: Dra. Nora B. Pappano. Laboratorio de Físico-Química, Departamento de Química. Universidad Nacional de San Luis. Chacabuco 917. (5700) San Luis, ARGENTINA. Fax: (+54-2652) 422644. E-mail: npappano@unsl.edu.ar
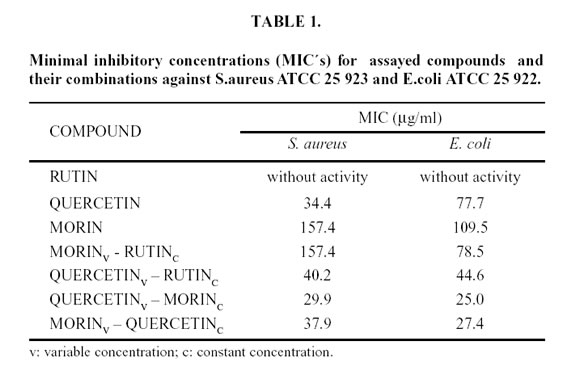
Abstract: In our previous studies, bacteriostatic action of flavonoids against Staphylococcus aureus ATCC 25 923 and Escherichia coli ATCC 25 922 was demostrated. In the present work synergism of their combinations in order to improve the bacteriostatic action against the same microorganisms was determined. The experiences were made in nutritive broth, maintaining constant one drug concentration (20 µg/ml) and increasing the other one. A turbidimetric kinetic method was used and by means of a mechanism previously proposed, the minimal inhibitory concentrations (MIC´s) of each flavonoid combination were determined. The MIC´s for assayed combinations against S. aureus were: variable morin - constant rutin: 157.44 µg/ml and variable quercetin - constant morin: 29.9 µg/ml. The values obtained against E. coli were: variable morin - constant rutin: 78.5 µg/ml; variable quercetin - constant rutin: 47.4 µg/ml; variable quercetin – constant morin: 25 µg/ml; variable morin - constant quercetin: 27.4 µg/ml.
Key words: Synergism; Flavonoids; Bacteriostatics; MIC
Introduction
Properties of flavonoid compounds in isolated form are widely known. The combinations of different flavonoids often improve considerably their biological activities, such as bactericidal and bacteriostatic action (Fukai et al., 1996; Fukai et al., 2002; Zeng et al., 1992), powerful antiviral activity (Dua et al., 2003; Wua et al., 2003), inhibition of colony formation in human leucemic cells (Fujita et al., 1997), clinical treatment of human ovary carcinoma (Kanasawa et al., 2003), amongst others. Polyphenolic compounds might inhibit carcinogenesis affecting molecular transformations in any of the initial, promotion or propagation stages (Yang et al., 2001). It has been suggested that tea-flavonoids hav chemiprotector properties against cancer (Vaidyanathan and Wallet, 2001), action against cardiovascular disease (Hertog et al., 1997; Riemersma and Rice-Evans, 2001; Yochum and Kushi, 1999), vascular protection against arteriosclerosis (Zenhua and Yuan, 1991), espasmolitic action in vitro and antidiarrhoeic effect (Sadraei et al., 2003), hepatoprotector effect (Yoshikawa et al., 2003), antioxidative activity (Bergman et al., 2003; Hodgson et al., 2002; Mariken et al., 2003; Miura et al., 2003; Moridani et al., 2003), relaxant muscular activity (Hosseinzadeh et al., 2003) and other therapeutic applications. Synergism of bacteriostatic action using combination of flavonoid compounds against Staphylococcus aureus ATCC 25 923 and Escherichia coli ATCC 25 922 was determined, using a turbidimetrickinetic method previously proposed (Pappano et al., 1990).
Materials and Methods
Microorganisms assayed
S. aureus ATCC 25 923 and E. coli ATCC 25 922 (purchased in American Type Culture Collection) maintained by successive subcultures in trypticase soy agar (BBL) at 4°C and by lyophilization. Compounds: flavonoids of high purity were used: quercetin (3,5,7,3´,4´-pentahydroxyflavone), morin (3,5,7,2´,4´-pentahydroxyflavone) and rutin (glycosidic form of quercetin), were all obtained from Sigma (Fig. 1).
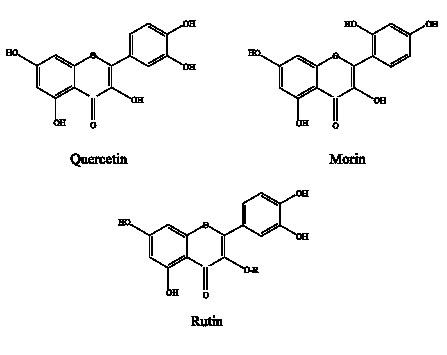
FIGURE 1. Structure of flavonoids
Turbidimetric-kinetic method
A 24 h culture of S. aureus ATCC 25 923 or E. coli ATCC 25 922 in nutritive agar was transferred to 30 ml of Müller-Hinton broth (Oxoid) and incubated during 18 h at 35 °C with permanent shake and it was used as inocule. Increasing quantities of one flavonoid (A) and a constant amount of another one (B) were added in erlenmeyers containing 100 ml culture media. There were other erlenmeyers which only contained increasing quantities of compound A. In addition, there was one erlenmeyer without flavonoid as control. Flavonoids A and B are structurally related. Each erlenmeyer was inoculated with 2 ml of inocule and then incubated in shaking Rosi 1000 culture chamber at 35°C and 180 rpm. Aliquots were extracted at 20 min intervals during 5 h, and transmittance (T) was determined at 720 nm using a Shimadzu UV 160 A spectrophotometer. These T values were related to the number of cfu/ml (colony forming units/ml) Nt, by means of the following expressions (Pappano et al., 1994):
| for S. aureus ln Nt = 27.4 - 10.3 . T | (1) |
| for E. coli ln Nt = 27.1 - 8.56 . T | (2) |
Results
The combinations of assayed compounds were efficient against S. aureus ATCC 25 923 and E. coli ATCC 25 922. The number of cfu/ml as a function of times was obtained from expression 1 or 2, depending on the microorganism.
Considering the curve of microbial growth
| ln Nt = µ. t + ln No | (3) |
where t: time in min; No: cfu/ml at the t = 0; Nt: cfu/ml at the t; µ: specific growth rate in min-1, the values of specific growth rates with increasing drug concentrations were obtained from the curve of ln Nt vs. t in the exponential phase.
According to previously exposed, results were satisfactory and agree with the mechanism of bacteriostatic inhibition before proposed (Pappano et al., 1990), where the specific growth rate (µ) varies according to the drug concentration in a linear relation:
| µ= µT - ki . C | (4) |
where: µ: specific growth rate (min-1); µT: specific growth rate without drug (min-1) (control); ki: specific inhibition rate (ml µg-1 min-1) and C: drug concentration (µg/ml).
Figures 2 and 3 are the graphical representations of equation 3 for quercetin and quercetin plus morin against S. aureus and E. coli, respectively.
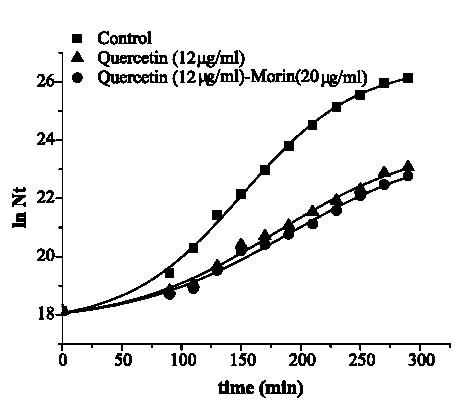
FIGURE 2. Growth of Staphylococcus aureus ATCC 25 923 in media containing quercetin and its combination with morin.
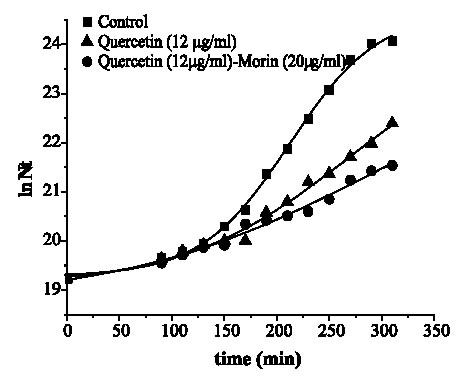
FIGURE 3. Growth of Escherichia coli ATCC 25 922 in media containing quercetin and its combination with morin.
Figures 4 and 5 show the linear dependency of µ with C (Equation 4), for the assayed compounds and their combinations against both microorganisms. Minimal inhibitory concentrations values (MIC´s) were calculated by extrapolation at µ= 0 and are listed in Table 1.
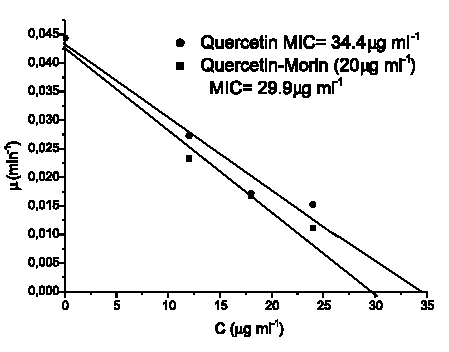
FIGURE 4. Graphical determination of MIC's (minimal inhibitory concentrations) by extrapolation at the abcis when µ= 0 for Staphylococcus aureus ATCC 25 923.

FIGURE 5. Graphical determination of MIC's (minimal inhibitory concentrations) by extrapolation at the abcis when µ= 0 for Escherichia coli ATCC 25 922.
Discussion
The results of the present work allow us to conclude that the noticeable diminution of MIC against E. coli in presence of a second flavonoid demonstrates synergism between studied compounds. This can be due to rutin which favors polar solutes entry, such as quercetin d morin, through structural membrane proteins d porines. Rutin binds to porines changing idimentional conformation exposuring the hydrophilic character of the pore. This makes an easier passage of polar flavonoids by diffusion. Flavonoid may be as anions, that case, their entry would be difficult through porines, formed by charged amino acids, opposing electrostatic repulsion to concentration gradient which impels its entry.
On the other hand, Gram positive bacteria exhibit teicoics, lipoteicoics and teicuronics acids into the cell wall and depending on its location and composition in the wall, these microorganisms present different resistance to bactericidal and bacteriostatic agents. In these cases, the transport defect through cytoplasmatic membrane s an intrinsic mechanism of resistance of some Gram positive and anaerobic bacteria. The penetration of these agents needs an active transport at cytoplasmatic membrane level and a force provided by electrical potential of electron chain. These mechanisms complicate entering agent to the action place, as probably happens in the case of flavonoids assayed against S. aureus, where synergism was practically null compared with observed against Gram negative bacterium E. coli.
Acknowledgements
The San Luis National University supported the present work.
References
Bergman M, Perelman A, Dubinsky Z, Grossman S (2003). Scavenging of reactive oxygen species by a novel glucurinated flavonoid antioxidant isolated and purified from spinach. Phytochemistry 62(5): 753-762. [ Links ]
Dua J, Hea Z, Jianga R, Yeb WC, Xua HXi, But PPH (2003). Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62(8): 1235-1238. [ Links ]
Fujita MM, Nagai M, Murata M, Kawakami K, Irino S, Takahara J (1997). Synergistic cytotoxic effect of quercetin and heat treatment in a lymphoid cell line (OZ) with low HSP70 expression. Leuk Res. 21(2): 139-45. [ Links ]
Fukai T, Cai BS, Horikoshi T, Nomura T (1996). Isoprenylated flavonoids from underground parts of Glycyrrhiza glabra. Phytochemistry 43(5): 1119-1124. [ Links ]
Fukai T, Marumoa A, Kaitoub K, Kandab T, Teradab S, Nomuraa T (2002). Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 73(6): 536-539. [ Links ]
Hertog M, Sweetnan P, Fehily A (1997). Antioxidant flavonol and ischemic heart disease in a Welsh population of men: the Caerphilly study. Am J Clin Nutr. 65: 489-494. [ Links ]
Hodgson JM, Croft KD, Murit A (2002). Regular ingestion of tea does not inhibit "in vivo" lipid peroxidation in humans. J Nutr. 132(1): 55-58. [ Links ]
Hosseinzadeh H, Ramezani M, Namjob N (2003). Muscle relaxant activity of Elaeagnus angustifolia L. Fruit seeds in mice. J Ethnopharmacol. 84(2-3): 275-278. [ Links ]
Kanazawa M, Satomib Y, Mizutania Y, Ukimuraa O, Kawauchia A, Sakaic T, Babad M, Okuyamad T, Nishinod H, Mikia T (2003). Isoliquiritigenin Inhibits the Growth of Prostate Cancer. European Urology 43(5): 580-586. [ Links ]
Mariken JT, Arts J, Sebastiaan Dallinga J, Voss HP, Haenen GR, Bast A (2003). A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chem. 80(3): 409-414. [ Links ]
Miura T, Muraoka S, Fujimoto Y (2003). Inactivation of creatine kinase induced by quercetin with horseradish peroxidase and hydrogen peroxide. Pro-oxidative and anti-oxidative actions of quercetin. Food Chem Toxicol. 41(6): 759-765. [ Links ]
Moridani MY, Pourahmad J, Bui H, Siraki A, OBrien PJ (2003). Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radical Biology and Medicine 34(2): 243-253. [ Links ]
Pappano NB, Centorbi OP, Ferretti FH (1990). Determinación de la concentración inhibitoria mínima a partir de parámetros cinéticos de crecimiento. Rev Microbiol. 21: 183-188. [ Links ]
Pappano NB, Centorbi OP, Ferretti FH (1994). Determination of the responsible molecular zone for the chalcones bacteriostatic activity. Rev Microbiol. 25: 168-174. [ Links ]
Riemersma RA, Rice-Evans RM (2001). Tea flavonoids and cardiovascular health. Q J Med. 94: 277-282. [ Links ]
Sadraei H, Asgharib G, Hekmattia A (2003). Antispasmodic effect of three fractions of hydroalcoholic extract of Pycnocycla spinosa. J Ethnopharmacol. 86(2-3): 187-190. [ Links ]
Vaidyanathan JB, Wallet T (2001). Transport and metabolism of the tea flavonoid epicatechin by the human intestinal cell line Caco-2. Pharm Res. 18(10): 420-425. [ Links ]
Wua JH, Wangb XiH, Yic YH, Lee KH (2003). Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorganic & Med Chem Letters 13(10): 1813-1815. [ Links ]
Yang CS, Landan JM, Huang MT, Newman HL (2001). Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann Rev Nutr 21: 381-406. [ Links ]
Yochum L, Kushi LH (1999). Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 149: 943-949. [ Links ]
Yoshikawa M, Xu F, Morikawa T, Ninomiya K, Matsuda H (2003). Anastatins A and B. New skeletal flavonoids with hepatoprotective activities from the desert plant Anastatica hierochuntica. Bioorganic & Medicinal Chemistry Letters. 13(6): 1045-1049. [ Links ]
Zeng L, Fukai T, Kaneki T, Nomura T, Zhang RY, Lou ZC (1992). Four new isoprenoid-substituted dibenzoylmethane derivatives, glyinflanins A, B, Cc, and D from the roots of Glycyrrhiza inflata. Heterocycles, 34(1): 85-97. [ Links ]
Zenhua D, Yuan C (1991). Inhibitory effect of China green tea polyphenol on the oxidative modification of LDL by macrophages. Med Sci Res. 19: 767-768. [ Links ]
Received on February 28, 2005.
Accepted on July 29, 2005.














