Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.30 n.1 Mendoza jan./abr. 2006
Single-channel response of hamster oocytes to ertilization with homologous spermatozoa
Leonor M.E. Ituarte*, Teresa B. Viera*, Teobaldo A. Saldeña*, Juan C. De Rosas**, Mabel Fóscolo**, Jorge E. Ibáñez* and Fernando D. Saraví*
* Área de Física Biológica, Departamento de Morfofisiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Casilla de Correo 33, 5500 Mendoza, Argentina.
** Instituto de Histología y Embriología «Dr. Mario H. Burgos», Departamento de Morfofisiología, CONICET-Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Casilla de Correo 56, 5500 Mendoza, Argentina.
Address correspondence to: Dr. Fernando D. Saraví. Area de Física Biológica, Departamento de Morfofisiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo. Casilla de Correo 33, (5500) Mendoza, ARGENTINA. Fax: (+54-261) 420 3288. E-mail: synerges@yahoo.com.ar
Abstract: Electrophysiological events occur early after fertilization, along with changes in intracellular Ca2+ concentration . Passive electrical parameters were determined in golden hamster oocytes by whole cell patch-clamp method. In separate experiments the effect of 4-aminopyridine on resting oocytes was tested. The single-channel patch clamp configuration was employed to assess the electrical response to fertilization with homologous sperm. Structure of oocytes submitted to patch clamp was evaluated with scanning electron microscopy and found to be preserved.
Oocyte diameter was 70.2 ± 2.2 µm; their resting parameters were: membrane potential 23.8 ± 0.8 mV; total membrane specific resistance 519.1 ± 94.6 Ω.cm2, and specific capacity 0.99 ± 0.03 µF.cm-2. Total membrane current was decreased by 42 % by 4-aminopyridine.
Control oocytes and oocytes exposed to sperm differed in their membrane currents in response to a voltage ramp clamping membrane potential from – 100 mV to + 100 mV. In both cases, currents were largest at the most negative potentials, but sperm-exposed oocytes had larger currents. Additionally, while in control oocytes the current was inward at negative potentials but outward at positive potentials, in the presence of spermatozoa oocytes was inward within the whole voltage range tested. This latter current may represent Ca2+ entry.
Key words: 4-Aminopyridine; Fertilization; Hamster oocyte; Patch-clamp; Membrane current
Introduction
Fertilization may be viewed as a highly specific form of cell-cell interaction, which results in the generation of a new member of the species. The first step of fertilization is gamete recognition, which, if successful, is followed by gamete binding and fusion. This singular, specialized cell-cell interaction involves reciprocal activation of the spermatozoon and oocyte, which results in metabolic activation of the latter and eventually, in resumption of meiosis. Two events mark the beginning of the oocyte response, namely cytosolic Ca2+ waves and a change of the oocyte membrane potential (Tosti and Boni, 2004).
Ca2+ is the major second messenger involved in oocyte activation. When mammalian oocytes are fertilized, an initial increase of oocyte cytosolic Ca2+ is followed by a number of Ca2+ waves or periodic oscillations in Ca2+ concentration (Melman and Kline, 1994; Nakada and Mizuno, 1998; Stricker, 1999; Sun and Nagai, 2003).
Concurrently with the beginning of the Ca2+ waves, although bearing a complex and probably species-specific relationship with them, the oocyte undergoes changes in transmembrane potential. Fertilization potentials differ among phyla. In non-mammalian oocytes, spermatozoa trigger a depolarization, while in mammalian species the characteristic response is a hyperpolarization caused by the opening of Ca2+-sensitive K+ channels. This hyperpolarizing response has been documented in several mammalian species by classical electrophysiological techniques (Miyazaki and Igusa, 1981, 1982) or whole-cell patch clamp (Gianaroli et al., 1994; Dale et al., 1996; Tosti et al., 2002). In the present study, the electrical response to fertilization in the golden hamster (Mesocricetus auratus) was studied by the single channel patch-clamp technique.
Materials and Methods
Chemicals and solutions
With the exceptions noted below, all chemicals were obtained from Sigma Chemical (St. Louis, MO, USA). Follicle-stimulating hormone was from Massone and human chorionic gonadotropin from Elea.
For oocyte and sperm isolation the HECM-3 medium was employed (Goud et al., 1998). The same medium was also used for the patch clamp experiments. The HECM-3 medium had the following composition: NaCl 113.8 mmol/L, KCl 3.0 mmol/L, CaCl2 1.9 mmol/ L, MgCl2 0.46 mmol/L, NaHCO3 15.0 mmol/L, HEPES 10.0 mmol/L, sodium lactate 3.5 mmol/L, L-glycine 2.0 mmol/L, L-hypotaurine 1.0 mmol/L, L-glutamine 0.2 mmol/L, Fraction V bovine serum albumin 3 g/L, penicillin 65 mg/L, and streptomycin 65 mg/L. For single channel recording, the micropipettes were filled with a medium resembling intracellular fluid, with the following composition: KCl 140.0 mmol/L, CaCl2 0.5 mmol/ L, EGTA 5.5 mmol/L, MgCl2 2.0 mmol/L, and HEPES 10.0 mmol/L.
Oocytes and spermatozoa
Hamsters were housed and handled in the Institute of Histology and Embryology according to the Medical Sciences School guidelines for the care and use of experimental animals.
Metaphase II oocytes were obtained from adulthamster females (5 to 6 week-old) submitted to sequential stimulation with i.p. follicle-stimulating hormone (20 U) followed 48 h later by i.p. human chorionic gonadotropin (Goud et al., 1998; see also Fleming and Yamaguchi, 1980; killed by cervical dislocation 24 h after the last injection. The Fallopian tubes were dissected and perfused with culture medium HCM-3 through a 28 G hypodermic needle to obtain the oocytes. In order to free oocytes from the cumulus oophorus and the zona pellucida, they were treated, respectively, with hyaluronidase and trypsin (both 1 mg/mL) for 15 min. Cumulus-free, zona pellucida-free oocytes were then washed three times and classified. Oocytes with abnormal morphology were discarded.
Spermatozoa were collected from 3 to 6 month-old male hamster of proven fertility. Cauda epididymes were carefully removed and placed in HECM-3 medium, cut into many pieces with fine scissors, allowing the spermatozoa to flow out spontaneously. Spermatozoa were collected and incubated at 37°C in a 5% CO2 in air atmosphere at 100% relative humidity for 2 h. Their motility was assessed. Sperm count was performed with a Neubauer hematocytommetry chamber.
Patch-clamp recording
Microelectrodes were made with heparin-free microhematocrit pipettes (Paralwall). To improve adherence of the tip to the oocyte membrane, pipettes were washed for 10 min in 1% NaOH at 95°C, then for 2 min in 5% HCl, washed thoroughly with bidistilled water at room temperature, and finally dried in a stove at 200°C for 30 min. Microelectrodes were prepared by stretching the pipettes with a two-step puller (Narishige, Model PP-83). The micropipettes thus obtained had their tips heat-polished with a microforge (Narishige, Model MF-830).
Microelectrodes intended for single channel patchclamp were insulated with a thick layer of hydrophobic resin Sylgard 184 (Dow Corning). For recording in the single-channel mode, micropipettes were filled with HECM-3, while for whole-cell recording micropipettes were filled with the intracellular fluid-like solution.
To minimize noise, the recording setup was surrounded by a grounded Faraday cage. A plastic Petri dish containing the oocytes was placed on the stage of an inverted microscope (Olympus, Model CK 2). The micropipette tip was positioned on the oocyte membrane with a pneumatic micromanipulator (Narishige, Model MHW-3). Afterwards negative (subatmospheric) pressurewas gently applied with a syringe to foster the development of a high resistance seal between the micropipettetip and the oocyte membrane. No additional maneuver was necessary for single- channel recording. For whole-cell recording mode, on the other hand, after the seal was formed a second suction step was performed to establish continuity between the oocyte cytoplasm and the micropipette contents.
The micropipette was connected to a high input impedance preamplifier (Dagan 8910) which fed the main amplifier (Dagan 8900). Signals were monitored with an oscilloscope and data were continuously recorded in a personal computer. Membrane seal resistance (Rs) was measured when a lineal signal was recorded. Applied stimuli were regulated through the Pclamp 6 software (Axon Instruments, Inc.).
Basal electrical properties and effect of 4-aminopyridine
Oocytes (n = 2 or 3 per experiment) were placed in a plastic Petri dish with 3 mL of HECM-3 solution exposed to air and placed on the stage of an inverted microscope Olympus CK 2. Experiments were carried out at room temperature under whole-cell configuation.
Total ionic current in response to square wave voltage clamp steps was determined. In separate experiments, recordings were also obtained after addition of the K+-channel blocker, 4-aminopyridine (30 mmol/L).
Fertilization with homologous spermatozoa
Oocytes were set as stated above at room temperature and patched in single channel configuration. After the seal was established, an aliquot of 30 µL of HECM- 3 solution with 106 spermatozoa/mL was added. In control experiments, 30 µL of HECM-3 without spermatozoa were added.
Oocytes exposed to spermatozoa and controls were submitted to the same patch-clamp protocol 5 min after the addition of sperm or vehicle. For single channel configuration, the membrane potential was clamped with a ramp waveform in the range from – 100 mV to + 100 mV, with a total duration of 2 500 milliseconds. Fifteen to 20 runs were performed for each oocyte.
Scanning electron microscopy
At the end of each experiment, oocytes with attached recording micropipette tips were fixed in 2.5% picric acid/paraformaldehyde and 2% glutaraldehyde in phosphate buffered saline (pH 7.4) at 4°C and left overnight at room temperature. Then they were repeatedly rinsed in phosphate buffered saline, mounted in mica grids covered with 1% gelatin, and dehydrated with series of increasing concentrations of ethanol and acetone. They were submitted to a critical point drying system (Sorval) and afterwards covered with gold with a sputter device (Balzer Union). Oocytes were observed with a Siemens ETEC scanning electron microscope at an accelerating voltage of 20 kV.
Statistical analysis
Differences between electrophysiological properties under basal conditions and after addition of 4- aminopyridine, and between single channel-currents in fertilized oocytes and controls were analyized with a two tailed Students t test for unpaired samples. Best fit for current-voltage curves were determined by a leastsquare algorithm. The current-voltage relationship was assessed by linear regression analysis. The best fit for the current-voltage relationship was determined by a Stephan Boltzmann approximation (Cui et al., 1997). Unless otherwise stated, results are expressed as mean ± standard error of the mean (SEM). A value of P < 0.05 was deemed significant.
Results
Basal properties and effect of 4-aminopyridine
Seal resistance was time-dependent, approaching a constant value after 10 min as shown by the high linear correlation coefficient between current and voltage reached at that time (r2 = 0.99). Recording a stable negative potential indicated that the oocyte cytoplasm had been accessed. The basal membrane properties of oocytes, as determined after the seal resistance was stabilized, are shown in Table 1. Single oocyte capacitance of was 20.00 ± 0.06 pF (n = 10). Membrane specific capacitance was calculated from the measured oocyte diameter, taking into account the known increase in surface area caused by microvilli (Zamboni, 1970; Suzuki et al., 1994). Figure 1 presents the effect of 4- aminopyridine on total ionic current and membrane conductance. It can be seen that both electrophysiological variables were consistently and significantly reduced by the drug.
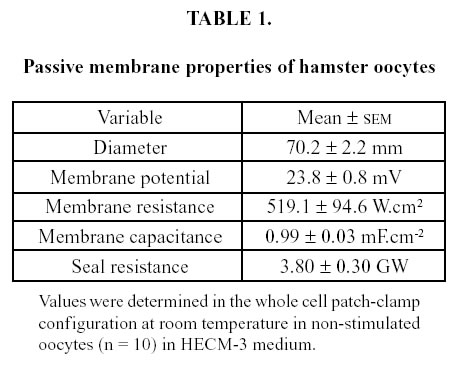

FIGURE 1. The response to a depolarizing voltage step of 10 mV from a holding potential of Ð70 mV was determined in the whole cell patch clamp configuration in separate experiments in oocytes under control conditions (n = 14) and in the presence of 4-aminopyridine (30 mmol/L; n = 14). Values are mean ± SD. * P = 0.001; § P = 0.036.
Fertilization with homologous spermatozoa
After stabilization, seal resistance for the single channel patch-clamp configuration was 30.0 ± 7.0 GΩ (n = 40). Oocytes exposed to spermatozoa had a larger current in response to the voltage clamp. Representa- tive recordings of a single run for a control oocyte, and of a single run for an oocyte exposed to spermatozoa, are shown in Figure 2. The larger current in the latter can be seen both at the beginning of the run, and smaller peaks, but still larger than those of the control, are seen near the end. Initial peaks correspond to inward current, while final peaks are outward current.
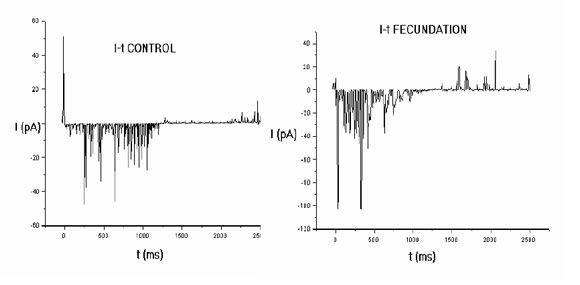
FIGURE 2. Recordings of current in single-channel patch-clamp configuration in an oocyte exposed to vehicle (left) and an oocyte exposed to spermatozoa (right). A ramp protocol with a total duration of 2 500 ms clamped the membrane potential in the range Ð100 to +100 mV. Membrane current is larger in the sperm-exposed oocyte. Notice the different position of the zero current point.
Figure 3 represents a whole experiment in an oocyte exposed to vehicle and an oocyte exposed to ermatozoa. The curve is the mean value of the relationship between membrane potential and membrane current. It can be seen that current is larger in the presence of sperm.
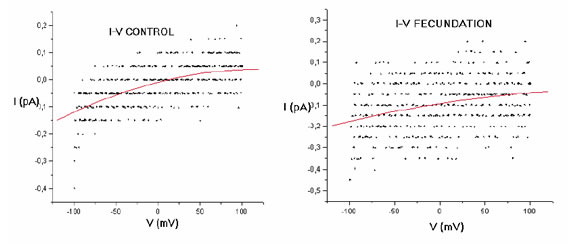
FIGURE 3. Current-voltage (I/V) relationship from two representative complete experiments (15 runs each) with a control oocyte (left) and a sperm-exposed oocyte (right). In both cases, the continous trace represents the mean I/V relationship calculated with a Boltzmann approximation method. Notice that the current crosses the zero value in the control situation, but remains negative (inwardly directed) throughout the clamping voltage range in the sperm-exposed oocyte.
A plot of experiments with five oocytes exposed to vehicle and with five oocytes exposed to sperm is shown in Figure 4. Again, in sperm-exposed oocytes the current is larger at negative membrane potentials. Additionally, while the current shows reversal from inward to outward at a membrane potential close to 0 mV in vehicle-exposed oocytes, in sperm-exposed oocytes it decreases but remains inwardly directed even at positive membrane potentials.
FIGURE 4. Current-voltage (I/V) relationship from experiments with control oocytes (left) and sperm-exposed oocytes (right); each n = 5. In both cases, the continous trace represents the mean I/V relationship calculated with a Boltzmann approximation method. Notice the different position of zero in the current axis.
Scanning electron microscopy
Zona-free oocyte surface appeared normal in scanning electron microphotographs. At the point here the micropipette tip was attached, a net, clear mark can be seen. Protrusions of the oocyte plasma membrane around this area may indicate the contact between the oolemma and the electrode tip which allowed the gigaohm seal (Fig. 5).

FIGURE 5. Scanning electron microphotograph of a cumulus-free, zona-free oocyte employed in a patch-clamp experiment. The area of contact with the microelectrode tip is clearly seen (arrow). Magnification X 3000.
Discussion
The morphological and electrophysiological characteristics of oocytes employed in the present tudy indicate both their structural and functional integrity. Scanning electron microscopy of oocytes after the electrophysiological experiments allowed checking the ultrastructural features of oocytes and the point of contact between the oocyte and the microelectrode tip.
Basal membrane electrophysiological properties found in the present study were in general agreement with those reported by Miyazaki and Igusa (1982) for hamster oocytes studied with conventional electrophysiological techniques. These properties included the resting potential and also membrane capacity, which was found to be about four times larger than those reported for most biological membranes (i.e., about 1 µF.cm-2) if a smooth spherical oocyte shape was assumed. This suggests that the oocyte microvilli increase the effective surface of the membrane about four times.
Experiments with 4-aminopyridine demonstrated that it reduced both membrane conductance and total membrane current in response to a small depolarizing voltage step. The effect of 4- aminopyridine on oocyte electrophysiology is remarkable, since this drug is known to block voltage gated outward K+ channels with fast activation and inactivation which have a role in muscle and nerve action potentials. Thus, 4-aminopyridine effects have been well characterized in excitable tissues, like the myocardium (Carmeliet, 1999) and the nervous system, where it even has been found to possess therapeutic properties in demyelinating disorders (Hayes, 2004). In contrast, to the best of our knowledge, 4- aminopyridine has not been previously shown to modify membrane current or total conductance in mammalian oocytes. Therefore, these effects await both independent confirmation and further exploration.
Previous reports on electrical events triggered by fertilization in mammalian oocytes (reviewed by Tosti and Boni, 2004) have employed either traditional electrophysiological techniques (Miyazaki and Igusa, 1981, 1982) or whole-cell patch clamp (Gianaroli et al., 1994; Dale et al., 1996; Tosti et al., 2002). Both of these approaches have an inherently low power to discriminate several components of a membrane current. On the other hand, the technique employed in the present paper yields information on the behavior of single channels, or at most a few channels, which are located within the membrane just below the electrode tip.
The voltage steps applied are well within the tolerance to electrical pulses reported for oocytes (Tan et al., 1996), thus ruling out possible electric breakdown of the oocyte membrane in the applied voltage clamping range. The single channel response of non-stimulated oocytes within the range tested was characterized by its low amplitude and its reversal at a clamped potential close to 0 mV. In other words, the current was reversed from negative (inward) at negative clamping potentials to positive (outward) at positive clamping potentials. This behavior of control oocytes suggests that it corresponds to non-specific currents (De Simone et al., 1998) carried through ionic channels of low selectivity.
The stimulation with homologous spermatozoa used in the present study has been previously shown to be highly effective for activation of zona-free hamster oocytes (Maleszewski et al., 1995). In the presence of spermatozoa, oocytes developed higher inward currents than controls. As in control oocytes, the highest currents were recorded at highly negative clamping potentials. However, in contrast with the non-stimulated oocytes the current remained inwardly directed even at positive clamping voltages. This indicates that it corresponds to ionic species with a highly positive electrochemical potential, like calcium ions. Together with previous evidence indicating entry of Ca2+ as an early activation phenomenon in mammalian oocytes (Melman and Kline, 1994; Nakada and Mizuno, 1998; Stricker, 1999; Sun and Nagai, 2003), this strongly suggests that the oocyte currents recorded in the presence of spermatozoa represent Ca2+ entry.
Acknowledgement
This work was supported by a grant from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Cuyo.
References
Carmeliet E (1999). Cardiac ionic currents and acute ischemia: From channels to arrhythmias. Physiol Rev. 79: 917-1017. [ Links ]
Cui J, Cox DH, Aldrich RW (1997). Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol. 109: 647-673. [ Links ]
Dale B, Fortunato A, Monfrecola V, Tosti E (1996). A soluble sperm factor gates Ca2+-activated K+ channels in human oocytes. J Assist Reprod Genet. 13: 573-577. [ Links ]
De Simone ML, Grumetto L, Tosti E, Wilding M, Dale B (1998). Non-specific currents at fertilization in sea urchin oocytes. Zygote 6: 11-15. [ Links ]
Fleming AD, Yamaguchi R (1980). Superovulation and superpregnancy in the golden hamster. Dev Growth Differentiat. 22: 103-122. [ Links ]
Gianaroli L, Tosti E, Magli C, Iaccarino M, Ferraretti AP, Dale B (1994). Fertilization current in the human oocyte. Mol Reprod Dev. 38: 209-214. [ Links ]
Goud PT, Goud AP, Rybouchkin AV, De Sutter P, Dhont M (1998). Chromatin decondensation, pronucleus formation, metaphase entry and chromosome complements of human spermatozoa after intracytoplasmic sperm injection into hamster oocytes. Hum Reprod. 13: 1336-1345. [ Links ]
Greenwald GS (1993). How does daily treatment with human chorionic gonadotropin induce superovulation in the cyclic hamster? Biol Reprod. 48: 133-142. [ Links ]
Hayes KC (2004). The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev. 10: 295-316. [ Links ]
Maleszewski M, Kline D, Yanagimachi R (1995). Activation of hamster zona-free oocytes by homologous and heterologous spermatozoa. J Reprod Fertil. 105: 99-107. [ Links ]
Melman LM, Kline D (1994). Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol triphosphate is enhanced after meiotic maturation. Biol Reprod. 51: 1088-1098. [ Links ]
Miyazaki SI, Igusa Y (1981). Fertilization potential in golden hamster eggs consists of recurring hyperpolarization. Nature (Lond). 290: 702-704. [ Links ]
Miyazaki SI, Igusa Y (1982). Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci USA. 79: 931-935. [ Links ]
Nakada K, izuno J (1998). Intracellular calcium response in bovine oocytes induced by spermatozoa and by reagents. Therionology 15: 269-282. [ Links ]
Stricker SA (1999). Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 211: 157-176. [ Links ]
Sun QY, Nagai T (2003). Molecular mechanisms underlying pig oocyte maturation and fertilization. J Reprod Dev. 49: 347-359. [ Links ]
Suzuki H, Yang X, Foote RH (1994). Surface alterations of the bovine oocyte and its investments during and after maturation and fertilization in vitro. Mol Reprod Dev. 38: 421-430. [ Links ]
Tan JH, Liu ZH, Sun XS, He GX (1996). Tolerance of oocyte plasma membrane to electric current changes after fertilization. Zygote 4: 275-278. [ Links ]
Tosti E, Boni R (2004). Electrical events during gamete maturation and fertilization in animals and humans. Human Reprod Update. 10: 53-65. [ Links ]
Tosti E, Boni R, Cuomo A (2002). Fertilization and activation currents in bovine oocytes. Reproduction 124: 835-846. [ Links ]
Zamboni L (1970). Ultrastructure of mammalian oocytes and ova. Biol Reprod Suppl. 2: 44-63. [ Links ]
Received on March 11, 2005.
Accepted on December 22, 2005.














