Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.30 no.1 Mendoza Jan./Apr. 2006
Synaptonemal complexes and XY behavior in two species of argentinian armadillos: Chaetophractus villosus and Dasypus hybridus (Xenarthra, Dasypodidae)
R. B. Sciurano, M.S. Merani, Jimena Bustos, and A.J. Solari
Centro de Investigaciones en Reproducción (CIR)- Facultad de Medicina, UBA. Paraguay 2155, (1121) Buenos Aires, Argentina.
Address correspondence to: Dr. Alberto J. Solari. Centro de Investigaciones en Reproducción (CIR), Facultad de Medicina, UBA. Paraguay 2155, (1121) Buenos Aires, ARGENTINA. E-mail: asolari@fmed.uba.ar
Abstract: Spermatocytes from the two armadillo species, C. villosus and D. hybridus were studied in microspreads for synaptonemal complexes (SCs) and in thin sections for electron microscopy (EM). The complete SC karyotype generally agrees with previous reports on mitotic chromosomes, except for the sex chromosomes. The X chromosome is submetacentric in both species and the Y is the shortest one in C. villosus and the second shortest in D. hybridus, and an extremely acrocentric one. A SC is formed along the total length of the Y chromosome, and this SC persists along all the pachytene substages. A single ecombination nodule (RN) is located in the region of the SC nearest to the attachment to the nuclear envelope. The lateral element (LE) of the X axis in the SC shows a wavy aspect in most of the SC length distant from the nuclear envelope. Nucleoli are attached to acrocentric or submetacentric bivalents, are visibly double in some cells , and in thin sections show an elaborate nucleolonema. Some differences in the XY are species-specific, as the higher degree of tangling and stronger heteropycnosis in D. hybridus. The effective, single crossover of the XY pair is highly localized, despite the permanence of a long tract of SC.
Key words: Xenarthra; XY chromosomes; Meiosis; Synaptonemal complexes
Introduction
Armadillos are included in the family Dasypodidae of the order Xenarthra, which radiated in the past from South America towards the Northern hemisphere. Xenarthrans, and especially armadillos, are interesting as living species related to a rich paleontological registry, which included among other, glyptodonts classically studied in Argentina. Furthermore, their relatively long ancestry and the special embryological features (polyembriony) in the genus Dasypus makes armadillos an interesting material for cytogenetic and genomic studies.
Recently, studies on the DNA of 12 of the 13 living Xenarthra genera (Delsuc et al., 2002) yielded strong evidence that Xenarthra is one of the four major clades of placental mammals, supporting the antiquity and basal position of this order. Furthermore, the suborder Cingulata, which comprises armadillos, has been analysed as regards molecular phylogeny, showing that the genus Dasypus and the genus Chaetophractus are the most phylogenetically distant (Delsuc et al., 2002), in agreement with the large reported differences in sperm size and structure (Cetica et al., 1993). Thus, an analysis of meiosis centered on species of these two genera may give clues to the whole suborder Cingulata, which in itself is one of the oldest radiations of eutherian mammals.
Karyological studies of armadillos are scanty, and most of them have been reviewed by Jorge et al. (1985). Beath et al. (1962) and Jorge et al. (1985) have reported karyotypes of species from the genus Dasypus, and a single study of the synaptonemal complexes has been reported on Dasypus novemcintus (Scavone et al., 2000).
The present paper is aimed to describe the synaptonemal complex karyotypes, the nucleolar features, and the behaviour of the sex chromosomes in males from two species, D. hybridus and C. villosus. The behaviour of the XY pair shows that despite a rare permanence of a full synaptonemal complex throughout pachytene, which stretches along the complete Y chromosome, there is a single recombination nodule (crossover) which is located towards the segment nearest to the nuclear envelope. In D. hybridus, nucleolar organizers (NORs) are located in a specific bivalent and elaborate nucleolar extensions are formed at early pachytene. The Y chromosome seems to be structurally identical in both species. The kinetochore of the Y is practically attached to the telomere, making the Y an extremely acrocentric chromosome, which is the smallest of the SC complement in C. villosus, and one of the two smallest elements in D. hybridus.
Materials and Methods
The animals were collected as part of a project on Xenarthran biology (PICTR 74, Foncyt).
For this work, two male specimens of Chaetophractus villosus, one collected at Pipinas, Provincia de Buenos Aires, the other collected at Trenel, Provincia de La Pampa, were trapped and carried to the laboratory under adequate care. One male specimen of Dasypus hybridus was collected at Pipinas, Provincia de Buenos Aires, and carried as above mentioned. The animals, which were also studied in their anatomy and other biological features, were hemicastrated or killed under anesthesia, the testes removed and divided into several pieces for different procedures. The sacrified animals were deposited in the mammalian collection of the Museo de la Universidad Nacional de La Plata (UNLP). For general testicular histology the pieces were fixed in Bouin fixative, embedded in paraffin and the sections were stained with hematoxylin-eosin. For fine structural cytology, the pieces were fixed in 2.5% glutaraldehyde in 0.2 M cacodylate buffer, pH 7.2 and then in OsO4, embedded in araldite and the thin sections were stained with uranyl acetate and lead citrate. For the specific analysis of synaptonemal complexes (SCs), a variant of the drying-down tecnique for SCs was used (Solari, 1998). The spermatocytes were stained either with 4% phosphotungstic acid (PTA) in ethanol or with silver nitrate (Howell and Black, 1980). Electron microscopy was done with a Siemens Elmiskop electron microscope, and magnifications were checked with a calibration grid (Pelco International, Redding, Ca., USA). Micrographs were scanned and the digital data were used for measurements with the software Micromeasure (Reeves and Tear, 2000).
Results
Synaptonemal complex karyotypes and their parallel mitotic karyotypes in C. villosus
The SC karyotype of C. villosus shows 29 autosomal SCs and the XY pair (Fig. 1). This SC karyotype corresponds to the already described chromosome number 2n =60 (Jorge et al., 1977, 1985). However, the order of the SCs was done according to relative length only, in order to have comparable sets from different cells. The location of centromeres kinetochores) was observed in PTA-stained specimens, and this procedure revealed 15 autosomal SCs with meta or- submetacentric components and 14 autosomal SCs with acrocentric elements. Thus, this classification agrees with that of Jorge et al. (1977). However, the identification of individual SCs in silver-stained nuclei, of #11-#13, #14- #16 and #17-#21 was not possible, and thus in these EM preparations there is ambiguity in the identity of some of the SCs. On the other hand, #1 is always identifiable as the longest SC (with sub-medial kinetochore) as well as the two smallest, acrocentric autosomal SCs (#28 and #29). Three nucleoli were observed in the majority of pachytene spermatocytes: in #6, #11 and in #25. The latter is an obvious small submetacentric, which presents its nucleolus in all the cells. More variable are the presence of nucleoli in crocentric #11 and the submetacentric #6.
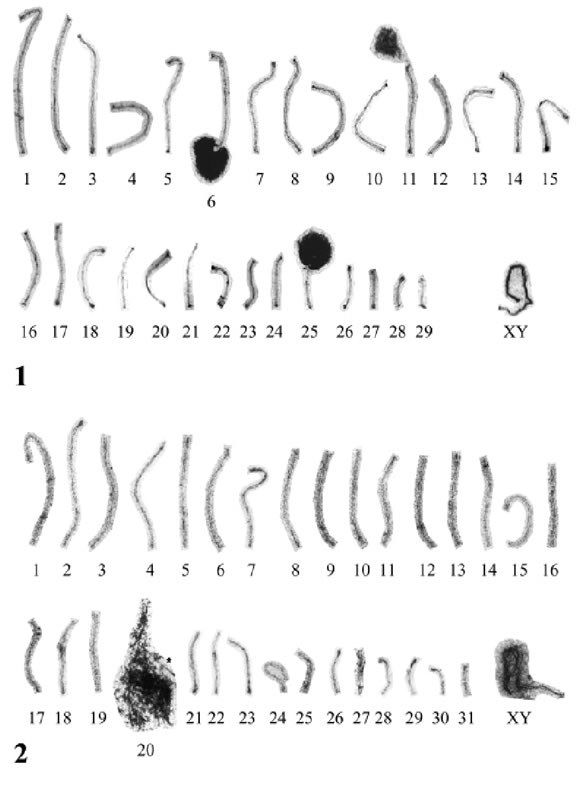
FIGURES 1 and 2. SC karyotypes of C. villosus (Fig. 1) and D. hybridus (Fig. 2). The three nucleoli of C. villosus are attached to their NOR-bearing SCs. In Fig. 2, the single SC bearing the nucleolus of D. hybridus is marked with an asterisk.
SC karyotype of D. hybridus
The SC karyotype of D. hybridus agrees with that reported for mitotic chromosomes, which is generally described as 2n =64. In fact, we find 31 autosomal SCs plus the XY pair, corresponding to a chromosome set of 2n=64 (Fig. 2). As no PTA-stained specimens for autosomes were studied in this species, we cannot comment on the relationship with mitotic karyotype morphology. On the other hand, a single SC was always observed attached to the single, large and filamentous nucleolus observed in pachytene nuclei. This is a small SC, longer than the Y axis and the axis of the smallest acrocentric SC. This NOR-containing SC could correspond to #22 in the related species D. novemcinctus, which has been classified by Barroso and Seuanez (1991) as submetacentric in that species.
It shoud be remarked that in D. hybridus the smallest acrocentric is shorter than the Y axis.
General features of the XY pairs in both species
The general appearance of the XY pair is very similar in both species. It forms a conspicuously heteropycnotic body at advanced pachytene stages and it shows two unequal axes (X axis and Y axis) in earlier stages (Figs. 3 and 4). The absolute length of the Y axis in C. villosus is 4.0 µm ± 0.8 µm (n=11). In D. hybridus the Y axis is 4.1 µm ± 0.6 µm (n=8).

FIGURES 3 and 4. Electron micrographs of silver-stained XY pairs of C. villosus (Fig. 3) and D. hybridus (Fig. 4) at early-mid-pachytene. The X-axis of C. villosus shows waviness or irregularities (arrowhead) in the upper part of the SC, and it splits into 4 strands in the differential region (arrow). The Y axis of D. hybridus shows the kinetochore (KY) as a dense protrusion before its ending. X 9,000.
Relative lengths and centromeric indexes (CIs) of the X and Y axes
The length ratio between the X axis and the Y axis is : X/Y = 4.5, n=5 in C. villosus, in early pachytene. This ratio is X/Y = 4.3, n=3 in D. hybridus, also in early pachytene.
The X axis can be measured only at early pachytene, as in later substages it forms a net-like, filamentous array in which it is not possible to perform measurements.
The general appearance and length of the Y axis are nearly identical in both species. In PTA-stained pachytene spermatocytes of C. villosus, the kinetochores of both axes are clearly established: the Y axis is extremely acrocentric (CI = 0.1) and the X axis is submetacentric (CI = 0.25) (Fig. 5).
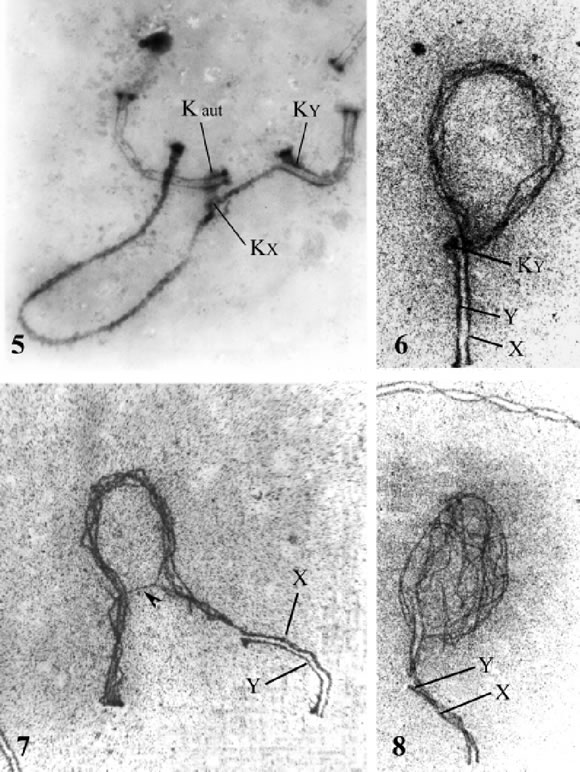
FIGURES 5-8. Electron micrographs of the XY pairs of C. villosus ( Figs. 5 and 7) and D. hybridus (Figs. 6 and 8). In Fig. 5, PTA staining allows the detection of the grey protuberances of the autosomal kinetochores (Kaut) and those of the X axis (Kx) and the Y axis (KY ). The kinetochore of the Y is almost terminal in both C. villosus (Fig. 5) and in D. hybridus (Fig. 6). Figs. 7 and 8 correspond to late pachytene. While in C. villosus there is a bridge in the differential part of the X-axis (arrowhead), in D. hybridus there is a net-like array. Figs. 5-6, X 12,000. Figs 7-8, X 9,000.
This was a surprising fact, as the X axis of C. villosus has been described as clearly acrocentric (Jorge et al., 1977). However, the centromere location in the X of C. villosus showes a clearly defined short arm, which is the one that pairs with the acrocentric Y (Fig. 5).
In D. hybridus the X is submetacentric and the Y, as in C. villosus, is extremely acrocentric and one of the two smallest of the set (Figs. 6 and 2).
Length of SC and variation with time
One of the striking peculiarities of the XY pair in both C. villosus and D. hybridus is the constancy of its length. This length is SC (XY) = 3.7 µm ± 0.8 µm (C. villosus, n=15) and 4.1 µm ± 0.6 µm (D. hybridus, n=8).
The length of the SC, which covers practically all the Y axis except its proximal end, is retained up to the more advanced pachytene substages (Figs. 7 and 8).
On the other hand, a few XY pairs at a very early stage (as shown by the total absence of axial differentiations in the X axis and incomplete synapsis in autosomes), showed the proximal part of the Y axis in unpaired condition. At very early pachytene or late zygotene, synapsis between the X and Y axes may be incomplete, as 6% of the Y length may be unpaired at these very early stages.
About half of the observed XY pairs showed either twisting or irregularities in one LE of the XY SC. The only affected axis in all cases was the X axis (Figs. 3 and 9). These irregularities correspond to the segment of the SC which is more distant from the nuclear envelope.
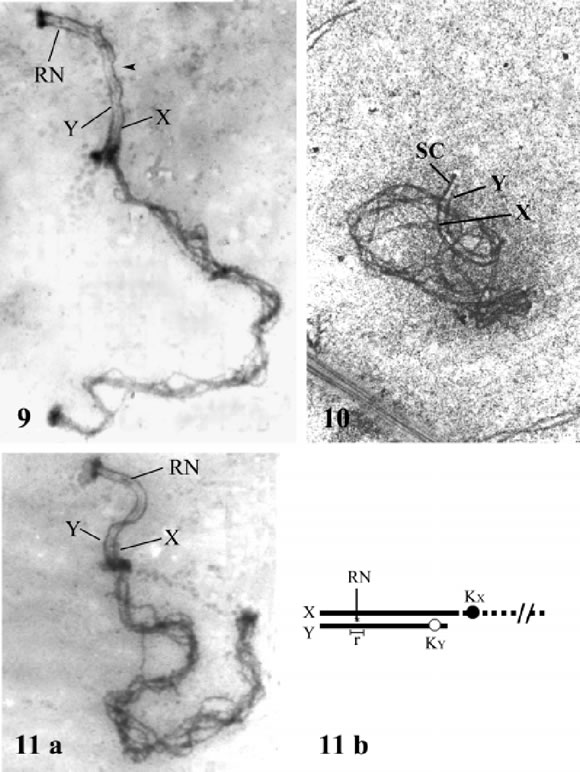
FIGURES 9-11. Permanency of the SC in the XY pair and location of the crossover (recombination nodule, RN). In Figs. 9 and 11a, the RN is located on the central region of the SC, near its upper end and before the waviness of the X axis (arrowhead in fig. 9). Fig. 10 shows the permanency of the full SC at very late pachytene in D. hybridus (silver staining). In Figure 11b the drawing at scale shows the average location of the RN (asterisk) and its narrow range (segment, below) as regards the extremely acrocentric Y of C. villosus. Figs. 9-11a, X 9,000.
Changes in the differential segment of the X axis in each species
In C. villosus the most striking differentiation of the differential segment of the X axis is its splitting into a number of strands, which is generally 4, (varying between 2 and 5). These strands seem to be branches and anastomoses of the original axis, which follow the same general direction. Some of these branches form bridges, which extend between the arms of the loop formed by the split X axis (Fig. 7).
There are some species-specific features in this differential segment, as the net-like array is more complex in D. hybridus, and the degree of heteropycnosis, as shown by the density of the surrounding cromatin, is much higher in D. hybridus when compared to C.villosus (Fig. 10).
Localization of recombination nodules (RNs, crossovers) in the XY pair
There is a single recombination nodule in the SC of the XY pair, which is located in a short segment near the attachment site of the SC with the nuclear envelope (Figs. 9 and 11a).
The mean location of the RN is 1.2 µm ± 0.3 (n = 8) from the nuclear envelope, and its range is 1.6 µm to 0.82 µm (Fig. 11b). This restriction of RN location has been found to agree with the location of a single crossover as shown by MLH1 immunolocalization (results not shown). Furthermore, the SC segment harboring the RN is always devoid of any of the irregularities or waviness above described (Figs. 3 and 9).
Thin sections
In thin sections, the XY body appears as a densely stained body attached to the nuclear envelope (Fig. 12a). This body shows an evenly spaced and compact chromatin, and sections of single axes or pieces of SC near the nuclear envelope.
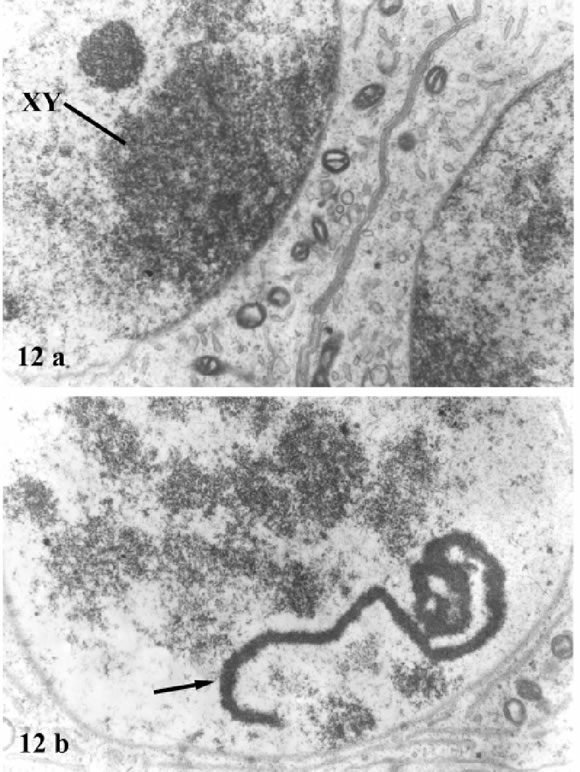
FIGURES 12a-12b. Thin sections of spermatocytes from D. hybridus. In 12a the XY body (XY) formed by the XY pair is shown as a mass of tightly packed chromatin attached to the nuclear envelope. In 12b one of the long, armlike expansions of the nucleolus is marked by an arrow. X 18,000.
In sections of D. hybridus, a large nucleolus is distinguished because of many long and curved expansions which can fuse to each other and have a finely granular structure (Fig. 12b).
Discussion
Validation of karyological data of Dasypodidae
Our results are in general agreement with the karyotypes described by Jorge et al. (1977) for C. villosus and D. hybridus. However, while these authors have claimed that the X chromosome of C. villosus is acrocentric and that it has "a prominent paracentromeric C+ band" (Jorge et al., 1977), we have shown that the X chromosome of C. villosus is submetacentric, with a clearly defined short arm which is the one that pairs with the acrocentric Y chromosome. The X axis of D. hybridus is also submetacentric. Thus, the X chromosome from both species studied here are very similar in morphology, and the Y chromosomes are also very similar in size, in the CI and in their behaviour (see Results). Our results also show that while the Y hromosome is the smallest element in C. villosus, it is not the smallest one in D. hybridus. This fact points to a lack of coherence in previous reports (Jorge et al., 1977, 1985) that state that there are no differences between the karyotypes of D. hybridus and that of D. novemcinctus, although in karyotypes from male specimens the latter species shows a longer Y chromosome. A re-examination of the karyotypes of males in largest samples of specimens seems to be needed to define this problem.
Significance of permanent total synapsis of the Y chromosome and differentiations in the unpaired segment of the X chromosome
In a previous report on a different species (D. novemvinctus) Scavone et al. (2000) have reported a complete synapsis between the X and Y chromosomes, and that the long arm of the X chromosome is the one that pairs with the small acrocentric Y.
Our results show that complete XY synapsis is shared with D. hybridus and C. villosus, two phylogenetically distant species of Cingulata, and thus this kind of full pairing may be characteristic of the family Dasipodidae. However, as shown in Results, in C. villosus it is the short arm of the submetacentric X chromosome the one that pairs with the Y axis and thus the specificity of pairing in the species D. novemcinctus remains to be tested. Despite some contrary claims (Scavone et al., 2000), full synapsis between the XY pair is transitory, to say the best, in most reported mammalian species (Solari, 1993). The permanent, total synapsis between the Y and the corresponding region of the X chromosome is actually seen only in the presently reported Xenarthran species and in the rodent Galea musteloides (Solari et al., 1993) in which there is also a peculiar dissociation of the SC in later pachytene substages.
On the other hand, the X-axis differentiations (splittings, mainly in C. villosus and net-like anastomosing, mainly in D. hybridus) are found in varying degrees of intensity, among many other mammalian species.
Presence of a pseudoautosomal region in the XY pair
The present results show that there is a localized region for the presence of recombination nodules in the XY pair of C. villosus (also tested with MLH1 foci). The recombinational region, commonly known as pseudoautosomal region of the XY pair of mammals (Solari, 1980, 1993) is here restricted to the distal 30% of the Y axis (see Results), near the nuclear envelope. Thus, if this narrow localization of recombination is also present in the related species D. novemcinctus, the end-to-end attachment of the X and Y hromosomes at first meiotic metaphase described by Barroso and Seuanez (1991) would be explained as the result of a terminal chiasma.
Evolutionary significance of the behaviour of sex chromosomes in Dasypodidae
Among mammals, there are three different patterns of behaviour of the meiotic sex chromosomes in the male sex (Solari, 1993). In the most primitive ones, the subclassPrototheria (monotremes), represented by only three living species (platypus and the echidnas) there is no XY body, and there is a complex sex-chromosome system including an X, a presumptive Y and 8 additional chromosomes that are associated by reciprocal translocations (Grutzner et al., 2004). On the other hand, the marsupials (metatherians) have an XY system, and an XY body in spermatocytes, but they lack a SC between the X and Y (Solari and Bianchi, 1975; Solari, 1993) and the X and Y axes are associated by a special "dense plate" (Solari and Bianchi, 1975) which has been shown to contain the protein SCP3 of the lateral elements of the SC (Page et al., 2003).
Among eutherian mammals the rule (with some exceptions, reviewed in Solari, 1993) is that there is an XY pair that forms an XY body in spermatocytes, and that has a single recombination nodule (crossover) which is highly localized near the nuclear envelope (Solari, 1993).
The present results add a new taxon to those already known to have recombination restriction in their heteromorphic sex chromosomes (Solari, 1993). Given the basal position of Xenarthra in the phylogenetic tree of eutherian mammals (see Introduction), it is challenging to assume if some of the features of the XY pair in Cingulata are primitive: that is, if full synapsis of an extremely acrocentric Y, in which the distal region is the only one in which gene recombination occurs, has some selective value, for instance, the inhibition of the proximal Y to engage in non-homologous recombinational events, that could give rise to a multiple sex-chromosome system. It is left for wider explorations of Xenarthran meiosis a conclusion on this matter. The localization of suitable markers, like the Sry gene and some of the markers in the conserved X chromosome would also be interesting for such phylogenetic analysis. The interest in the basic genetics of these species is furthered by the recognition that armadillos are natural hosts of Trypanosoma cruzi type II, the prevalent agent of Chagas disease (Yeo et al., 2005).
Acknowledgements
This work was supported by a grant from FONCYT ( PICTR 00074) and from Conicet (2173 and 2174) and UBACYT (M008 and M051). MSM and AJS are members of the Carrera del Investigador (Conicet). The helpful assistance of Dra. M.I. Pigozzi with MLH1 immunolocalization, and that of Lic. M. I. Rahn and C. Deparci in the preparations is gratefully thanked.
References
Barroso CML, Seuanez H (1991). Chromosome studies in Dasypus, Euphractus and Cabassous genera (Edentata: Dasypodidae). Cytobios 68: 179-196. [ Links ]
Beath MM, Benirschke K, Brownhill LE (1962). The chromosomes of the nine-banded armadillo, Dasypus novemcinctus. Chromosoma 13: 27-38. [ Links ]
Cetica P, Sassaroli L, Merani MS, Solari A (1993). Compararative spermatology in Dasypodidae (Priodontes maximus, Chaetophractus villosus & Dasypus hybridus). Biocell 18: 89- 103. [ Links ]
Delsuc F, Scally M, Madsen O, Stanhope MJ, de Jong WW, Catzeflis FM, Springer MS, Douzery EJP (2002). Molecular phylogeny of living Xenarthrans and the impact of character and taxon sampling on the placental tree rooting. Mol Biol Evol 19: 1656-1671. [ Links ]
Grutzner F, Rens W, Tsend-Ayush E, El-Mogharbel N, O'Brien PC, Jones RC, Ferguson-Smith MA, Marshall Graves JA (2004). In the platypus a meiotic chain of ten chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432: 913-917. [ Links ]
Howell WM, Black DA (1980). Controlled silver staining of nucleolus organizing regions with a protective colloidal developer: A one-step method. Experientia 36: 1014-1015. [ Links ]
Jorge W, Meritt DA, Benirschke K (1977). Chromosome studies in Edentata. Cytobios 18:157-172. [ Links ]
Jorge W, Orsi-Souza AT, Best R (1985). The somatic chromosomes of Xenarthra. In: The evolution and ecology of armadillos, sloths and vermilinguas, G. G. Montgomery (ed.). Smithsonian Institute Press, Washington. [ Links ]
Page J, Berrios S, Rufas JS, Parra MT, Suja JA, Heyting C, Fernandez-Donoso R (2003). The pairing of the X and Y chromosomes during meiotic prophase in the marsupial species Thylamis elegans is maintained by a dense plate developed from their axial elements. J Cell Sci 116:551-560. [ Links ]
Reeves A, Tear J (2000). Micromeasure for Windows, version 3.3. Free program distributed by the authors through Internet. Link: http//:www.colostate.edu/Depts/Biology/Micromeasure [ Links ]
Scavone MDP, Oliveira C, Bagagli E, Foresti F (2000). Analysis of the synaptonemal complex of the nine-banded armadillo, Dasypus novemcinctus. Genet Mol Biol 23: 613-616. [ Links ]
Solari AJ (1980). Synaptonemal complexes and associated structures in microspread human spermatocytes. Chromosoma 81: 315-337. [ Links ]
Solari AJ (1993). Sex chromosomes and sex determination in vertebrates. pp 109-135. CRC Press, Boca Ratón. [ Links ]
Solari AJ (1998). Structural analysis of meiotic chromosomes and synaptonemal complexes in higher vertebrates. In: Berrios M, ed., Nuclear structure and function. Methods in Cell Biol. Vol 53, pp 235-256. Academic Press, San Diego. [ Links ]
Solari AJ, Bianchi NO (1975). The synaptic behaviour of the X and Y chromosomes in the marsupial Monodelphis dimidiate Chromosoma 52: 11- 25. [ Links ]
Solari AJ, Merani MS, Burgos MH (1993). Dissociation of the synaptonemal complex in the XY body of Galea musteloides (Rodentia, Caviidae). Biocell 17: 25-37. [ Links ]
Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, Miles GAJ, Lopez E, Gonzalez N, Patterson JS, Gaunt MW, Arias AR, Miles MA (2005). Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol, 35: 225-233. [ Links ]
Received on May 16, 2005.
Accepted on October 17, 2005.














