Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.30 n.1 Mendoza ene./abr. 2006
Characterization of the Brain-pituitary axis in pejerrey Odontesthes bonariensis
G.M. Somoza, L.A. Miranda, L.G. Guilgur, and P.H. Strobl-Mazzulla
Laboratorio de Ictiofisiología y Acuicultura. IIB-INTECH. CC 164. (B7130IWA). Chascomús, provincia de Buenos Aires, Argentina.
Address correspondence to: Gustavo M. Somoza. IIB-INTECH, Camino de Circunvalación Laguna, Km 6 (B7130IWA) Chascomús, ARGENTINA. E-mail: somoza@intech.gov.ar
Key words: Brain; Pituitary; Reproduction; Sex differentiation.
The pejerrey fish (Odontesthes bonariensis), a large atherinid native from the inland waters of Buenos Aires Province, is considered one of the most emblematic fresh water fish species of Argentina (López et al., 2001). Although it is an important commercial and game fish, its culture has not been well developed in Argentina. Besides, some reports have demonstrated the existence of problems in intensive pejerrey aquaculture: early sexual maturation, the presence of spawning asynchrony between females and low growth rates (Strüssmann, 1989; Strüssmann et al., 1993).
During the last years the interest of the laboratory has been focussed on the study of the reproductive physiology and the mechanisms involved during sexual determination/ differentiation in pejerrey, not only from a basic point of view but also keeping attention to the potential application of the knowledge on the development of pejerrey aquaculture.
In this context, the goal of the present work was to summarize the findings on the characterization of the brain-pituitary-gonadal axis in Odontesthes bonariensis.
Gonadotropin-releasing hormone
Gonadotropin-releasing hormone (GnRH) is a key neurohormone that regulates reproduction in all groups of vertebrates. This decapeptide is synthesized by neurons in different regions of the brain being its best known function the regulation of gonadotropins production and release by the pituitary gland (Seeburg et al., 1987).
Today it is well known that the brain of vertebrates expresses at least two GnRH variants. However, in teleosts fish there is a growing number of species in which three different GnRH forms have been identified (Somoza et al., 2002a, b; Lethimonier et al., 2004). Up to date 14 different GnRH forms have been described in vertebrates and they are usually called using the common name of the species in which they were first described (Adams et al., 2002; Lethimonier et al., 2004). In those bony fish species expressing three variants in their brain, these GnRH forms are distributed over specific brain areas: chicken GnRH-II (cGnRH-II) is expressed by neurons of the midbrain tegmentun (MT), salmon GnRH (sGnRH) is localized in the terminal nerve ganglion (TNG) and the third species-specific form is mainly found in the anterior preoptic area (POA) and the hypothalamus (Fernald and White 1999; González-Martínez et al., 2001).
Also in pejerrey fish, three different GnRH variants have been found: sGnRH, cGnRH-II, and pejerrey GnRH (pjGnRH), a novel member of the GnRH family isolated for the first time in this species (Stefano et al., 1997; Montaner et al., 2001). The immunostaining of pejerrey brain sections showed that sGnRH is mainly expressed by neurons located at the TNG, pjGnRH at the nucleus preopticus periventricularis (NPP) of the POA, and cGnRH-II at the MT (Stefano et al., 2000). In addition, only pjGnRH was detected in fibers entering the anterior pituitary gland (Stefano et al., 1997, 2000; Somoza et al., 2002a). More recently the full length of the cDNAs encoding for the three GnRH precursors of pejerrey was obtained by the RACE method (Rapid Amplification of cDNA Ends). The analysis of these sequence showed that the cDNAs of pjGnRH, cGnRH-II and sGnRH have 441, 530 and 516 bp, having an open reading frame of 297, 252 and 276 bp, respectively (Guilgur et al., 2003; GenBank accession #AY744689, #AY744687, #AY744688). It was also demonstrated the expression of different GnRH forms in other pejerrey organs such as: gonads, eye, kidney, spleen, liver, gill and olfactory epithelium suggesting novel functions for these peptides (Guilgur et al., 2003).
When GnRH precursors from pejerrey are subjected to a phylogenetic analysis they can be grouped following the scheme proposed by Vickers et al. (2004): pjGnRH in group one (GnRH I), containing GnRH variants located at the POA and related to the control of the pituitary gland; cGnRH-II from pejerrey in group two (GnRH II), located in neurons of the MT and sGnRH from pejerrey in group three (GnRH III), in neurons of the anterior brain (Fig. 1). In this framework it is also important to note the high percentages of identity observed between pjGnRH and seabrean GnRH (sbGnRH) precursors of different Acanthopterigian species, reinforcing the hypothesis that pjGnRH emerged from the gene codifying for sbGnRH (Montaner et al., 2001; Somoza et al., 2002b).
FIGURE 1. Phylogenetic tree of GnRH preprohormones from different teleost species. The tree was constructed using a Clustal V multiple sequence alignment program (DNAstar). The deduced amino acid sequences were obtained from the GenBank.
Gonadotropins
Gonadotropins (GtHs) are pituitary heterodimeric glycoproteins consisting of a common a-subunit and a b-subunit that confers hormonal specificity. Teleost fish GtHs are structurally related to the tetrapod folliclestimulating (FSH) and luteinizing (LH) hormones and are also important in the regulation of gametogenesis and sexual maturation (Quérat et al., 2000; Swanson et al., 2003).
In the last years, the genes codifying for both gonadotropins have been cloned and their expression studied in many bony fish species (see Weltzein et al., 2004). As a first step to study the function of GtHs in pejerrey, the full length of the cDNAs encoding for FSH and LH -b subunits were obtained by RACE (Miranda et al., 2003a; 2004). The cDNA for FSH-SS and LH-SS have 466 and 558 bp with an open reading frame of 351 and 450 bp, respectively (Miranda et al., 2004). Comparing these sequences with the deduced aminoacidic sequence of other teleost species it can be inferred that pejerrey FSH-b has a signal pepide of 15 amino acids (aa), and a mature peptide of 102 aa with a putative N-linked glycosylation site at residue Asn 12. Pejerrey LH-b is composed by a signal peptide of 32 aa, a mature peptide of 118 aa and a putative N-linked glycosylation site at residue Asn 10. Pejerrey LH-b, as well as all vertebrate species, has conserved the position of 12 cysteines and 1 putative N-linked glycosylation site (Swanson et al., 2003; Weltzein et al., 2004). Pejerrey FSH-b, also contains 12 cysteines; however, the position and number of cysteines in FSH-b varied among teleosts (Swanson et al., 2003).
The aminoacid sequence of pejerrey FSH-b compared to other teleost fish sequences varies between 33.1- 66.9% and is lower than that observed for LH-b subunits (46.3-72.4%). These results follow the pattern indicating that the primary structure of LH-b subunits has been better conserved than FSH-b during teleosts evolution, suggesting a rapid divergence of the FSH-b subunit (Quérat et al., 2000). Comparison studies also showed that pejerrey GtHs are closer to other Actinopterigian GtHs (Fig. 2).
FIGURE 2. Phylogenetic tree of b-GtHs subunits from different teleost species. The tree was constructed using a Clustal V multiple sequence alignment program (DNAstar). The deduced amino acid sequences were obtained from the GenBank.
Sex differentiation
Odontesthes bonariensis has a strong thermolabile sex determination (TSD). The proportion of females gradually varies from 100% at 15-19ºC to 0% at 29ºC when the larvae are kept at different temperatures between the 1-5th after hatching (Strüssmann et al., 1997). These results make pejerrey an interesting model to study the effects of the temperature on the mechanisms of sex determination and differentiation in teleost species.
In this species, the ovarian differentiation was first recognized at week 7 at 17ºC and at week 4 at 24ºC, meanwhile testicular differentiation began at week 7 at 24ºC and at week 4 at 29ºC. Blood vessels can be first observed at the gonads at week 6, 4, and 3 at 17, 24, and 29°C, respectively (Strüssmann et al., 2004). Thus, blood vessels are formed just before or concomitant with the first signs of commitment to either sex at all temperatures. These observations are consistent with the concept of extragonadal regulation of sex differentiation in this species.
During development, either FSH and LH, are expressed in the anterior pituitary gland during the process of gonadal determination/differentiation (Miranda et al., 2001a) and both GtHs show a clear correlation with the increase or decrease of the number of GnRH neurons located at the POA and the identification of immunoreactive GnRH fibers in the pituitary gland (Miranda et al., 2001a, 2003b). Also, the number of FSH, LH and GnRH neurons at the POA varies according to the incubation temperature suggesting a correlation with TSD. Taking together, these results suggested that the synthesis and release of GtHs may be regulated by GnRH before or just at the moment of sex determination showing that the hypothalamic-pituitary-gonadal axis is active during the time of sex determination in this species (Miranda et al., 2003; Strüssmann et al., 2004). It can also be hypothesized that GtHs regulate the production of sex steroids in the gonadal primordia and/or in the interrenal gland during the sexual determination/ differentiation period (Strüssman et al., 2004).
Control of pejerrey reproduction in captivity
A series of studies were performed in order to optimize pejerrey reproduction in captivity, including the increase of sperm volume and the synchronization of spawning by hormonal treatments.
In pejerrey, the sperm volume obtained by stripping of males either in natural conditions or captivity is very scarce (Strüssmann, 1989; Miranda et al., 2001b), being necessary several males to fertilize the eggs produced by a single female (Calvo et al., 1977).
In pejerrey males it was possible to increase sperm volume by environmental and hormonal treatments practically during all the year around without affecting the sperm concentration and motility. These treatments included: increase of the light phase of photoperiod, human chorionic gonadotropin (hCG), heterologous pituitary extracts and GnRH superactive analogues (GnRHA) injection (Miranda et al., 2001b; 2005). In order to compare all hormonal treatments, an experiment using the doses previously demonstrated to induce a 5 time increase of expressible milt was performed. The treatment with sGnRH-A was the most effective. However, the use of hCG is recommended because of its effectiveness to stimulate pejerrey spermiation in low doses, its low cost and availability (Fig. 3).
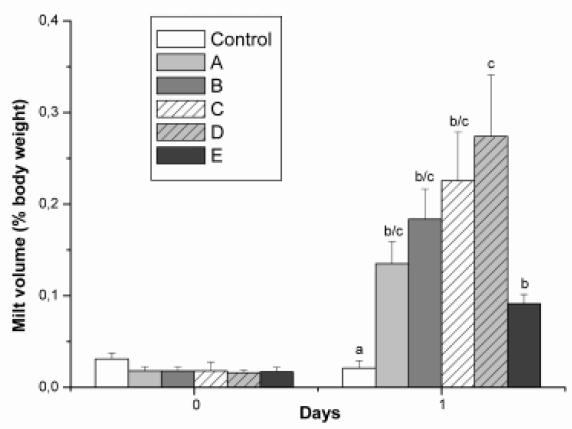
FIGURE 3. Volume of expressible milt in Odontesthes bonariensis 24 hours after the administration of 0.7% saline solution (Control), hCG: 78 IU/kg (A), carp pituitary extracts: 10mg/kg (B), salmon pituitary extracts: 10 mg/kg (C), sGnRH-A: 5 mg/Kg (D) and mGnRH-A: 2.5 mg/kg (E). Values represent mean ± SEM, n=8 per treatment. Significant differences between treatments respect control group are indicated by different superscript letters (P< 0.05).
In the case of females, a commercial sustained release system (Ovaplant, Syndel, Vancouver, Canada) was used in order to synchronize spawning. Pejerrey females (250 g) in late vitellogenesis with no signals of final oocyte maturation, were intraperitoneally implanted either with pellets having 75 mg of a sGnRH-A or blank pellets. The first groups of eggs were obtained after 60 hours in the sGnRH-A group and one week later it was shown that 82% of sGnRH-A implanted females had spawned meanwhile none of the control implanted females spawned during that period.
Although much work is necessary in order to find easier and non expensive ways to control pejerrey reproduction in captivity, these results demonstrated that is possible to use hormonal manipulation to induce reproduction in this species. In this context, it is important to think that even though the use of endocrine agents can be use to induce reproduction in this species, the study of environmental and maintenance conditions can also be effective to synchronize reproduction in pejerrey.
Acknowledgements
This work was supported in part by grants of ANPCYT, Argentina (Pict 01-04424, Pict 01-12168 and Pict Redes 00528), Fundación Antorchas; CONICET PEI# 6439 to G.M.S and PEI#6438 to LAM and Ministry of Education, Culture, Sports, Science and Technology of Japan (#15201003) to Carlos Strüssmann. The authors also thank Jim Powell from Syndel International Inc for his generous donation of Ovaplant.
References
Adams BA, Vickers ED, Warby C, Park M, Fischer WH, Craig AG, Rivier JE, Sherwood NM (2002). Three forms of gonadotropin- releasing hormone, including a novel form, in a basal salmonid, Coregonus clupeaformis. Biol Reprod 67: 232-239. [ Links ]
Calvo J, Morriconi ER, Zavala Suárez JE (1977). Fenómenos reproductivos en el pejerrey (Basilichthys bonariensis). II. Proporción de sexos y desplazamientos reproductivos. Physis 36: 135-139. [ Links ]
Fernald RD, White RB (1999). Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol 20: 224-40. [ Links ]
González-Martínez D, Madigou T, Zmora N, Anglade I, Zanuy S, Zohar Y, Elizur A, Muñoz-Cueto JA, Kah O (2001). Differential expression of three different Prepro-GnRH (Gonadotrophin- releasing hormone) messengers in the brain of the European sea bass (Dicentrarchus labrax). J Comp Neurol 429: 144-155. [ Links ]
Guilgur LG, Miranda LA, Somoza GM (2003). Characterization of three GnRH cDNA sequences in the pejerrey fish Odontesthes bonariensis. Fish Physiol Biochem 28: 39-40. [ Links ]
Lethimonier C, Madigou T, Muñoz-Cueto JA, Lareyre J, Kah O (2004). Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish.Gen Comp Endocrinol 135: 1-16. [ Links ]
López HL, Baigún CR, Iwaskiw JM, Delfino RL, Padín OH (2001). La cuenca del Salado: Uso y posibilidades de sus recursos pesqueros. Editorial de la Universidad de La Plata. La Plata. pp 76. [ Links ]
Miranda LA, Strussmann C, Somoza GM (2001a). Immunocytochemical identification of GTH1 and GTH2 cells during the temperature-sensitive period for sex determination in pejerrey, Odontesthes bonariensis. Gen Comp Endocrinol 124: 45-52. [ Links ]
Miranda LA, Escaray RU, Bustingorry JF, Somoza GM (2001b). Effects of photoperiod and human chorionic gonadotropin (hCG) administration on spermiation in pejerrey Odontesthes bonariensis. Rev Arg Prod Anim 21: 95-105. [ Links ]
Miranda LA, Guilgur LG, Somoza GM (2003a). Molecular cloning of cDNAs encoding FSH-b and LH-b subunits in the pejerrey fish, Odontesthes bonariensis. Fish Physiol Biochem 28: 101-102. [ Links ]
Miranda LA, Strobl Mazzulla PA, Strussmann CA, Parhar Ishwar, Somoza GM (2003b). Gonadotropin-releasing hormone neuronal development during the sensitive period of temperature sex determination in the pejerrey fish, Odontesthes bonariensis. Gen Comp Endocrinol 132: 444-453. [ Links ]
Miranda LA, Somoza GM, Strussmann CA (2004). Caracterización molecular de FSH-b y LH-b en el pejerrey bonaerense. Congreso Conjunto de Sociedades Biomédicas. Mar del Plata, Argentina, November 16-20. In Spanish. [ Links ]
Miranda LA, Cassara MC, Somoza GM (2005). Increase of milt production by hormonal treatment in the pejerrey fish Odontesthes bonariensis. Aquac Res 36: 1473-1479. [ Links ]
Montaner AD, Park MK, Fischerw, Craig AG, Chang JP, Somoza GM, Rivier JE, Sherwood NM (2001). Primary structure of a novel gonadotropin-releasing hormone (GnRH) variant in the brain of pejerrey (Odontesthes bonariensis). Endocrinology 142: 1453-1460. [ Links ]
Quérat B, Sellouk A, Salmon C (2000). Phylogenetic analysis of the vertebrate glycoprotein hormone family including new sequences of sturgeon (Acipenser baeri) b-subunits of the two gonadotropins and the thyroid-stimulating hormone. Biol Reprod 63: 22-228. [ Links ]
Seeburg PH, Mason AJ, Stewart TA, Nikolics K (1987). The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog Horm Res 43: 69-98. [ Links ]
Somoza GM, Lescheid DW, Miranda LA, Lo Nostro FL, Magliulo- Cepriano L, Montaner AD, Schreibman MP, Rivier JE, Sherwood NM (2002a). Expression of pejerrey gonadotropin- releasing hormone (pjGnRH) in three orders of fish. Biol Reprod 67: 1864-1871. [ Links ]
Somoza GM, Miranda LA, Strobl-Mazzulla PH, Guilgur LG (2002b). Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cel Mol Neurobiol 22: 589-609. [ Links ]
Stefano AV, Canosa LF, D'Eramo JL, Fridman O, Affanni JM, Somoza GM (1997). GnRH molecular variants in the brain and pituitary gland of pejerrey, Odontesthes bonariensis (Atheriniformes). Chromatographic and immunological evidence for the presence of a novel molecular variant. Comp Biochem Physiol 118: 335-345. [ Links ]
Stefano AV, Aldana M, Affani JM, Somoza GM (2000). Gonadotropin- releasing hormone (GnRH) neuronal systems in the pejerrey Odontesthes bonariensis (Atheriniformes). Fish Physiol Biochem 23: 215-223. [ Links ]
Strüssmann CA (1989). Basic studies on seed production of pejerrey Odontesthes bonariensis. Doctoral Thesis, Tokyo University of Fisheries, Tokyo, 351pp. [ Links ]
Strüssmann CA, Chon NB, Takashima F, Oshiro T (1993). Triploidy induction in an atherinid fish, the pejerrey (Odontesthes bonariensis). Progr Fish Cult, 55: 83-89. [ Links ]
Strüssmann CA, Saito T, Usui M, Yamada H, Takashima F (1997). Thermal thresholds and critical period of thermolabile sex determination in two atherinid fishes, Odontesthes bonariensis and Patagonina hatcheri. J Exp Zool 278: 167-177. [ Links ]
Strüssmann CA, Karube M, Miranda LA, Patiño R, Somoza GM, Uchida D, Yamashita M (2004). Methods of sex control in fishes and an overview of novel hypotheses concerning the mechanisms of sex differentiation. In: "Fish genetics and aquaculture biotechnology". TJ Pandian, CA Strussmann, MP Marian Eds. Oxford and IBH Publishing/Science Publishers, New Delhi, India/Enfield, USA, 65-79.
Swanson P, Dickey JT, Campbell B (2003). Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28: 1-7. [ Links ]
Vickers ED, Laberge F, Adams B, Hara T, Sherwood NM (2004). Cloning and Localization of Three Forms of Gonadotropin- Releasing Hormone, Including the Novel Whitefish Form, in a Salmonid, Coregonus clupeaformis. Biol Reprod 70: 1136- 1146. [ Links ]
Weltzein FA, Andersson E, Anderser O, Shakchian-Tabrizi K, Norberg B (2004). The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comp Biochem Physiol 137: 447-477. [ Links ]
Received on April 10, 2005.
Accepted on June 1, 2005.














