Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.30 no.1 Mendoza Jan./Apr. 2006
Silversides in South Brazil: Morphological and ecological aspects
M. A. Bemvenuti
Laboratório de Ictiologia, Departamento de Oceanografia, Fundação Universidade Federal do Rio Grande, C. Postal 474, Rio Grande, 96201-900, RS, Brazil.
Address correspondence to: Dr. M.A. Bemvenuti. Laboratório de Ictiologia, Departamento de Oceanografia, FURG, C.Postal 474, Rio Grande, CEP 96201-900, RS, BRAZIL. E-mail: docmab@furg.br
Key words: Silversides; Odontesthes; Brazil; Morphology; Multivariate.
The coastal plain of the Rio Grande do Sul state (RS) in southern Brazil, has an enormous lagoon complex in its southern portion formed by Patos Lagoon, Mirim Lagoon, Mangueira Lagoon and several other smaller lakes. This system was originated from successive transgression and regression cycles, which took place since the upper Pleistocene (Vilwoock, 1984). Patos Lagoon is a huge chocked lagoon (10.360 area km2), with an estuarine area comprising 10% of the total area in its southern portion, where one can find small, oligohaline shallow water embayment less than 3m maximum depth (Delaney, 1965).
Mirim Lagoon has an area of 3.749 km2, but only 2.382 km2 is located on Brazilian's territory because the remaining belongs to Uruguay. Mirim Lagoon communicates with Patos Lagoon through the São Gonçalo channel (Delaney, 1965).
Other small coastal lagoons are located along the coastal line, and are locally known as "lakes in rosary". The larger one is the Mangueira Lake, a closed lake with an area of approximately 800 km2. Its northern portion is connected with the Taim wetland, which is formed by small lakes (Jacaré, Nicola, Flores). The other lakes are shallow (from 2 to 3 m) and are situated in the north portion of the Rio Grande do Sul state (Fig. 1).

FIGURE 1. Area of distribution of silverside species in southern Brazil.
Ten species of silversides, comprising the genus Odontesthes, occur along the RS coastal plain (Bemvenuti, 2002; Malabarba and Dyer, 2002). In Patos Lagoon estuary and its adjacent marine coastal area occurs O. argentinensis (Valenciennes) and O. incisa (Jenyns), whereas in the freshwater habitats of Patos- Mirim lagoon system can be found O. bonariensis (Valenciennes), O. humensis De Buen, O. retropinnis (De Buen), O. aff. perugiae Evermann and Kendall and O. mirinensis Bemvenuti. In contrast, O. bicudo Malabarba and Dyer, O. piquava Malabarba and Dyer and O. ledae Malabarba and Dyer are endemic of a chain of small shallow lakes spread along the RS north coastline. Most of Odontesthes species co-occur in the same habitats and they are characterized by morphological similarities, which hinder their taxonomic identification.
Comparisons among multivariate morphometric techniques
There has been a lot of controversy regarding morphological differences among organisms due to the lack of agreement about which morphometric variables are more adequate and which kind of mathematic transformations of these variables are better to distinguish different forms.
Morphometric variables obtained by traditional measurement techniques do not take into account allometric variation or the different growth stages, which occur naturally in the organisms. Consequently, these data usually bring error to the analyses, leading to misleading interpretation and, ultimately, do not represent the real form of the organisms Bookstein et al. (1985). According to these authors, the use of traditional morphometric measurements can lead to more difficulties when differentiating among forms due to: (1) longitudinal measurements along the main body axis produces repetitive information in the same direction, which results in a unequal coverage of the body form, (2) utilization of maximum and minimum distances, which are not homologous from one individual to another, (3) utilization of long measurements, crossing several growth units (e.g., bones) and short ones, which contain local information.
Currently, the selection of characters that represent the whole body utilize short measure distances that connect anatomical points (homologous among individuals) (e.g., truss network) (Strauss and Bookstein, 1982). The different forms are distinguished in all directions, which compensate for random errors of measurements. Beside the description of growth and allometric patterns inside populations, the technique can discriminate among groups of organism that change in size and shape.
A comparison between traditional measurements and truss network (Fig. 2A, B) was investigated between two silverside species Odontesthes bonariensis and O. humensis through a principal component analysis. The first main component have been interpreted as variation in size, which results from the different growth stages in fishes, whereas the second component represents changes in shape of the organisms (Bookstein et al., 1985).

FIGURE 2. (A) Conventional set of distances measures used in fish morphometrics of O. humensis; (B) a truss networks of distances measures connect ten homologous landmarks of O. bonariensis (for explanation of this data set see Bemvenuti and Rodrigues, 2002).
In the comparative study of the different forms between two species it is interesting to separate the information obtained with size due to the differential growth showed by the organisms. Therefore, the data were log transform and also adjusted to remove the size effect factor (Burnaby, 1966). The two series of measurements used during adjust and no-adjust analyzes showed the formation of two groups. However, truss network analysis results in a better differentiation between O. bonariensis and O. humensis, probably because the method enhances the discrimination of the forms with the utilization of cross measurements not considered in the traditional method (Fig. 3A, 3B). The differential pattern between both groups was also observed by the position of values in the graph, which was located nearer one another in the truss network when compared with the traditional method (Fig. 3B; Bemvenuti and Rodrigues, 2002).

FIGURE 3. Principal components analysis of O. bonariensis (n=26) and O. humensis (n=25), in the space of the first two adjusted principal components (PC1, PC2), for (A) 25 traditional measures and (B) 21 distances measures with truss network.
Morphological differentiation among species of the perugiae complex1
Due to the large variation in the abiotic factors in the estuarine area of the Patos Lagoon it is possible to find O. argentinensis and O. mirinensis in the same location inside the estuary, but in distinct conditions of salinity. Odontesthes argentinensis shows preference for the lower reaches of the estuary, whereas O. mirinensis can be found in the northern reaches of the estuary, near the São Gonçalo Canal and Mirim Lagoon. This pattern brings difficult to the taxonomic identification of these species in the estuarine region. With the principal component analysis it was possible to distinguish O. argentinensis and O. mirinensis groups, but differences were not observed in body proportion between both species (Fig. 4). Only the meristic character of number of lower gill rakers shows differentiation: O. argentinensis 20 and 24 gill rakers, and O. mirinensis - 24 and 29 gill rakers (Bemvenuti, 1995, 2000, 2002).

FIGURE 4. Principal components analysis of O. mirinensis (n=41) and O. argentinensis (n=35), in the space of the first two adjusted main components (PC1, PC2), for 21 measures with truss network.
In the freshwater sites, the silversides O. aff. perugiae e O. mirinensis2 also were discriminated through the form (Fig. 5), being possible to observe a superficial variation in snout length, head and jaw (Fig. 6). In O. perugiae, the head is prominent (24.3% cp), the snout is salient (9,1% cp) and the maxilla is located ahead of the mandible. These proportions are relatively larger in O. peruagiae when compared with O. mirinensis, where the head is smaller (22.5% cp), the snout is shorter (6.9% cp) and the maxilla and mandible are in the same anterior projection (Bemvenuti, 1995, 1997, 2002).

FIGURE 5. Principal components analysis of O. aff. perugiae (n=52) and O. mirinensis (n=44), in the space of the second and third components (PC2, PC3), for 25 traditional measures.
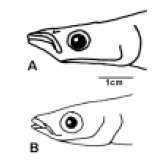
FIGURE 6. Lateral view of the head of O. perugiae (A) e O. mirinensis (B).
Description of the bone structure for the perugiae complex
A compared description of some bone structures was investigated to incorporate new data for the differentiation of the following species O. mirinensis, O. perugiae and O. argentinensis3. The teeth in the vomer are placed in three large plates in O. perugiae, in a medium size plate in O. argentinensis, and absents in O. mirinensis. The accessory canal of the infraorbital 1 (IO1) has two external pores in O. mirinensis, one anterior and intern pore and other posterior and external pore in O. perugiae, and it is absent in O. argentinensis. The infraorbital 2 (IO2) has a main dorsal canal and four long accessory canals with six pores in O. argentinensis, three long canals with five pores in O. mirinensis, and two short canals in O. perugiae.
The premaxilla shows posterior process upward, lengthier than higher in O. mirinensis, slim, large and straight in O. perugiae positioned upward in O. argentinensis. The mandibular sensory canal of the dentary shows seven pores in O. mirinensis and O. perugiae, and six in O. argentinensis. The tooth plate of the endopterygoid is numerous in O. mirinensis and O. argentinensis, whereas it is reduced a few and scattered tooth in O. perugiae. The preopercle sensory canal of the horizontal shaft has four large pores in O. mirinensis, three large pores in O. argentinensis, and no pore in O. perugiae due to the absence of bridges among them. The vertical shaft is totally open in O. mirinensis, with a wide opening in O. perugiae and with two openings in O. argentinensis. The hemal funnel, expansion of the hemal arch, start at the 24a vertebra in O. argentinensis, whereas in O. mirinensis, O. perugiae, and in the other freshwater silversides, the hemal funnel start at the 27a vertebra. The estuarine species has the same number of precaudal and caudal vertebrae, whereas the freshwater species has a larger number of pre-caudal vertebrates (Dyer, 1997; Bemvenuti, 2005).
Geographical differentiation in O. argentinensis
Odontesthes argentinensis is an estuarine-resident (Chao et al., 1985) showing a complete life-cycle inside the estuary. Morphological variations were observed among specimens occurring in the estuarine area and in the marine adjacent region (Fig. 7; Bemvenuti, 1995; 1997).

FIGURE 7. Principal components analysis of O. argentinensis, obtained in the marine coastal (n=26 - Praia do Cassino) and estuarine area (n=26 - Lagoa dos Patos; n=5 - Tramandai; n=3 - Lagoa da Concei‹o), in the space of the first two adjusted components (PC1, PC2), for 25 traditional measures.
Based on isoenzyme electrophoresis analysis, Beheregaray and Levy (2000) also observed similar patterns of genetic divergence between both populations. These authors suggested that the colonization of the estuary occurred when a segment of the marine silverside group managed to stay in the estuarine area, leading to the generation of differences among these groups. Based on molecular analysis, Beheregaray and Sunnucks (2001) suggested that all species of the genus Odontesthes are derived from the marine group. Therefore, this geographical area supports two resident silverside populations: one inside the estuarine area of Patos Lagoon and other in the adjacent marine area (Bemvenuti, 2000; Beheregaray, 2000; Beheregaray and Levy, 2000).
Bio-ecological aspects of the silversides
Feeding
The occurrence of several silverside species along the coastal lagoons of southern Brazil suggest changes in the feeding modes and prey selection to avoid diet overlap and, therefore, allowing their co-existence in the same system. The feeding behaviour is a fundamental step in understanding the ecology of one species and its place in the food web.
Species occurring in the same location probably feed upon different kinds of food, or occupy different habitats or utilize distinct resources in different time frames. Such species usually show diets with low overlap, which can be attributed to differences in mouth morphology and/or feeding behaviour. Diet composition must be related with morphological structures linked to feeding, such as the form, position and mouth size, and form and number of gill rakers (Wootton, 1990).
The feeding habit of O. humensis is benthos carnivore, preying upon mainly on mollusks Heleobia sp. (61.4% frequency of occurrence FO), Corbicula fluminea (58%), Neocorbicula limosa (17.4%) and coleoptera insects (18,8%). In lower frequency can be found diptera insects, crustacean Paleomonetes argentinus and fishes (Rodrigues and Bemvenuti, 2001). In spite of being a pelagic organism, the species browse for food in the bottom as well, making it different from other silverside species. The predominance of Heleobia sp, in comparison of other mollusks found in the diet, can be related with the high abundance of this prey in the study area and, probably, due to its low size. The majority of the shells of adults from C. fluminea and N. limosa were found crushed, whereas the shells of the young individuals were found as whole shells.
The form and number of the short and thick gill rakers (20-24= 4-6+15-19), as well the form and type of the tooth in the pharyngeal plates4 (molariforms in the center and conic in the edges), can be related with the zoo-benthos diet. The pharyngeal plates play a role in crushing mollusk shells and/or arthropod carapaces when they are too large. Shells of young C. fluminea, N. limosa and, in part Heleobia sp, are not crushed by the pharyngeal plates but are swallowed as entire pieces. The occurrence of coleopteran insects, as a prey item, can be explained by the high temperatures during the sample collection. This item also showed larger size them mollusk preys.
In the same environment, O. bonariensis shows zoobenthos habit preying upon mollusks, isopods, shrimps, tanaidacea, insects and small fishes. Among mollusk items, showed preference in frequency of occurrence by the gastropod Heleobia sp. (31%), bivalves Corbicula fluminea (16.7%) and Neocorbicula limosa (7.1%). Odonthestes aff. perugiae shows preference by mollusks, isopods, insect larvae, followed by tanaidacea and copepods. The food items with higher weight in the stomachs were the mollusk Corbicula fluminea (48% of the total weight) and isopods (31%), followed by insect larvae (8%), Hymenoptera insects (2%) and fishes remaining (5%) showing lower weight.
In the estuarine region, O. argentinensis's juvenile shows preference for zooplankton food items, whereas adults show preference for benthic preys. Juveniles prey upon copepods (63,8%) and diptera insects (35.5%), whereas adults feed on tanaidacea Kalliapseudes schubartii (35.6%), amphipod (22%) and polychaete Laeonereis acuta (25.7%) (Bemvenuti, 1990).
Reproduction
The estuarine specie O. argentinensis shows spawning season between late winter and early spring (June and November with a peak in September). The size of first maturation (L50) is 230mm TL (Bemvenuti, 1987). Recent information suggests a reduction of the maturation size in females (162mm TL in the estuary and 179mm TL in the marine area).
The freshwater species O. humensis and O. bonariensis spawn in the coastal lagoons during the early winter and spring. Odontesthes bonariensis spawns between May and July, whereas O. humensis between July and September (Kleerekoper, 1945; Moraes, 1991). The overlap in the spawning season increases the chances of combination among theses species, which favors hybridization. Preliminary karyotype studies5 suggest there is chromosomal affinity between both species, with chromosomes showing high similarity in number and morphology.
In spite of the extent literature about O. bonariensis and, in a less extent, about O. humensis, there is no record regarding hybridization with these species or others South American silversides. Recently it was developed its hybridization in laboratory with the help of natural populations of O. humensis e O. bonariensis6. The sampled individuals were analyzed by principal component. Two groups were observed (Fig. 8); one group showing a relationship between the natural population of O. humensis with hybrids which male progenitors were O. humensis, and the other group showed a relationship between the natural populations of O. bonariensis with hybrids which male progenitors were O. bonariensis. Such affinity shows the morphological similarity among the hybrids and the species of the male progenitor.
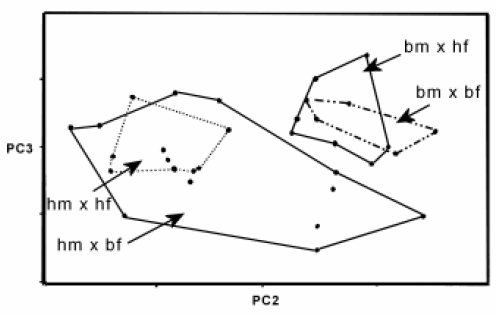
FIGURE 8. Principal components analysis of O. bonariensis (n=6 - bm x bf), O. humensis (n=5 - hm x hf), hybrids of O. bonariensis male and O. humensis female (n=8 - bm x hf), hybrids of O. humensis male and O. bonariensis female (n=14 - hm x bf) in the space of the second and third components (PC2, PC3), for 21 distances measures with truss network.
1. In the lagoons located in the south portion of the Rio Grande do Sul, the perugiae complex is comprised by the freshwater silversides O. perugiae, O. mirinensis, and the estuarine O. argentinensis. These three species are morphologically much closed and the morphometric and meristic characters are no sufficient tools to distinguish among them (Bemvenuti, 2002). According to Beheregaray et al. (2002), these silverside species were originated from the marine ancestor O. argentinensis.
2. Odontesthes perugiae is a specie restrict to rivers. Its occurrence is cited for Rio da Prata and Rio Paraná floodplain, Argentina (Marrero 1950), and including Uruguai and Negro rivers. Beheregaray et al. (2002) believed that the individuals from Uruguai river, RS are O. perugiae, and the individuals from the coastal lagoons as a new specie, O. aff. perugiae. Odontesthes mirinensis is restricted to coastal lagoons in the southern RS, Mirim, Mangueira e Lagoa dos Patos (Tapes, Camaquã, Guaíba river). Its high abundance is recorded in the Mirim Lagoon, with many juvenile individuals being captured in the Canal São Gonçalo Channel, Pelotas, which makes the connection between Mirim lagoon and Lagoa dos Patos (Bemvenuti, 1995).
3. The complete bone description for all Odontesthes species can be found in Bemvenuti (2005).
4. The pharyngeal plates are distributed in four pairs, being three located in the upper portion of the mouth and the other in the lower portion. The three plates of the upper portion have different size; one is large and oval shape with molariform tooth showing different size and the other two are smaller, external with small, slim and conic tooth. The lower plate is large, has a triangular shape, showing in the center several tooth of medium and small size, with a molariform shape, and in its edges show few small, slim and conic teeth.
5. Personal communication of G. Born (DCMB-FURG, Rio Grande, Rio Grande do Sul, Brasil).
6. Hybridization developed in laboratory in partnership with J. Pouey (UFPel, Pelotas, Rio Grande do Sul, Brazil).
References
Beheregaray LB (2000). Molecular evolution, biogeography and speciation of the Neotropical fish genus Odontesthes. PhD thesis, Macquarie Univ., Sydney, 211p. [ Links ]
Beheregaray LB, Levy JA (2000). Population genetics of the silverside Odontesthes argentinensis (Teleostei, Atherinopsidae): evidence for speciation in an estuary of southern Brazil. Copeia, Lawrence, pp. 441-447. [ Links ]
Beheregaray LB, Sunnucks P (2001). Fine-scale genetic structure, estuarine colonization and incipient speciation in the marine silverside fish Odontesthes argentinensis. Molecular Ecology, Oxford, 10: 2849-2866. [ Links ]
Beheregaray LB, Sunnucks P, Briscoe DA (2002). A rapid f ish radiation associated with the last sea-level changes in southern Brazil: the silverside Odontesthes perugiae complex. Proc R Soc London B 269: 65-73. [ Links ]
Bemvenuti MA (1987). Abundância, distribuição e reprodução de peixes-rei (Atherinidae) na região estuarina da Lagoa dos Patos, RS, Brasil. Atlântica, Rio Grande, 9(1): 5-32. [ Links ]
Bemvenuti MA (1990). Hábitos alimentares de peixes-rei (Atherinidae) na região estuarina da Lagoa dos Patos, Rs, Brasil. Atlântica, Rio Grande, 12: 79-102. [ Links ]
Bemvenuti MA (1995). Odontesthes mirinensis, um novo peixerei (Pisces, Atherinidae, Atherinopsinae), para o extremo sul do Brasil. Revta Bras Zool Curitiba, 12(4): 881-903. [ Links ]
Bemvenuti MA (1997). Relações morfológicas e osteológicas entre Odontesthes perugiae e O. mirinensis (Pisces: Atherinidae, Atherinopsinae). Atlântica, Rio Grande, 19: 113-131. [ Links ]
Bemvenuti MA (2000). Diferenciação geográfica do peixe-rei Odontesthes argentinensis (Atherinopsidae), no extremo sul do Brasil, através da morfometria multivariada. Atlântica, Rio Grande, 22: 71-79. [ Links ]
Bemvenuti MA (2002). Diferenciação morfológica das espécies de peixes-rei Odontesthes Evermann and Kendall (Osteichthyes, Atherinopsidae) no extremo sul do Brasil: morfometria multivariada. Revta bras Zool, Curitiba, 19(1): 239-249. [ Links ]
Bemvenuti MA (2005). Osteologia comparada entre as espécies de peixes-rei Odontesthes Evermann and Kendall (Osteichthyes, Atherinopsidae), do extremo sul do Brasil. Revta bras Zool, Curitiba, (in press). [ Links ]
Bemvenuti MA, Rodrigues FL (2002). Análise comparativa entre técnicas morfométricas aplicadas a Odontesthes bonariensis (Valenciennes) e Odontesthes humensis De Buen (Osteichthyes, Atherinopsidae). Revta bras Zool, Curitiba, 19(3): 789-796. [ Links ]
Bookstein FL, Chernoff B, Elder R, Humphries J, Smith G, Strauss R (1985). Morphometrics in evolutionary biology. Acad Nat Sci Philad, Philadelphia, (15): 1-277. [ Links ]
Burnaby TP (1966). Growth invariant discriminant functions and generalized distances. Biometrics, Arlington, 22: 96-110. [ Links ]
Chao LB, Pereira LE, Vieira JP (1985). Estuarine fish community of the Patos Lagoon (Lagoa dos Patos, RS, Brazil) - A baseline study. Ch.20, 26p. In: Yánez-Arancibia (ed) Fish community ecology in estuaries and coastal lagoons. Towards na ecosystem integration. UNANN, México 900p. [ Links ]
Delaney PJV (1965). Fisiografia e geologia da superfície da Planície Costeira do Rio Grande do Sul. UFRGS, Porto Alegre, 6, 195p. [ Links ]
Dyer BS (1997). Phylogenetic revision of Atherinopsinae (Teleostei, Atherinopsidae), with comments on the systematics of the South American freshwater fish genus Basilichthys Girard. Miscellaneous Publications, Museum of Zoology, University of Michigan, Ann Arbor, USA (185): 1-64. [ Links ]
Kleerekoper H (1945). O peixe-rei. Serv Infor Min Agric Rio de Janeiro, 98p. [ Links ]
Malabarba LR, Dyer BS (2002). Description of three new species of the genus Odontesthes from the rio Tramandai drainage, Brazil (Atheriniformes: Atherinopsidae). Ichthyol Explor Freshwaters, München, 13(3): 257-272. [ Links ]
Marrero A (1950). Flechas de Plata. Atherinidos argentinos pejerreys y laterinos. Ed. Breitman Buenos Aires, 175p. [ Links ]
Moraes PRR (1991). Peixe-rei: maturação e reprodução em laboratório. Revta da UCPel, Pelotas 1(1): 60-73. [ Links ]
Rodrigues FL, Bemvenuti MA (2001). Hábito alimentar e osteologia da boca do peixe-rei, Odontesthes humensis de Buen (Atheriniformes, Atherinopsidae) na Lagoa Mirim, Rio Grande do Sul, Brasil. Revta bras. Zool 18(3): 793-802. [ Links ]
Strauss RE, Bookstein, FL (1982). The truss: body form reconstructions in morphometrics. Syst. Zool. Washington, 31(2): 113-135. [ Links ]
Vilwoock JA (1984). Geology of the coastal province of Rio Grande do Sul, southern Brazil: a syntesis. Pesquisas, UFRGS, Porto Alegre, 16: 5-50. [ Links ]
Wootton RJ (1990). Ecology of Teleost Fishes. New York, Chapman and Hall, 404 p. [ Links ]
Received on February 3, 2005.
Accepted on May 11, 2005.














