Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.30 no.2 Mendoza May/Aug. 2006
CAS role in the brain apoptosis of Bufo arenarum induced by Cypermethrin
M. F. Izaguirre, M. N. Vergara, and V. H. Casco.
Laboratorio de Microscopía, FI-Bioingeniería, UNER, Ruta 11, CC 47, suc. 3, (3100), Paraná, Entre Ríos, Argentina.
Address correspondence to: Dr. Victor Hugo Casco. Laboratorio de Microscopía. FI-Bioingeniería, UNER. C.C. 47 Suc. 3. (3100) Paraná, Entre Ríos, ARGENTINA. Fax: (+54-343) 475077. E-mail: vcasco@bioingenieria.edu.ar
Abstract: CAS might have a key role in the apoptosis induced by toxins, acting as anti-apoptotic factor, stimulating the cellular proliferation and the cell contact stabilization. To start to elucidate their role in the brain apoptosis of Bufo arenarum induced by cypermethrin (CY), the expression patterns of CAS and several cell adhesion molecules (CAMs) were established.
Bufo arenarum tadpoles of the control and acute bioassay survival at different doses (39, 156, 625 and 2,500 µg CY/L) and times (24, 48, 72 and 96 h) of CY treatment were fixed in Carnoy, embedded in paraffin and sectioned. CAS and CAMs expression was determined by immunofluorescence and immunohistochemistry, respectively.
When the bioassay starts, CAS increases suggesting a proliferative or regenerative effect, but decreases when the doses and/or the biocide exposure time increases, suggesting compromise of the cellular cycle control and trigger of an apoptotic wave. However, these neurotoxic mechanisms should not involve degradation of N-cadherin and α-catenin, in contrast of β-catenin and axonal N-CAM180, at least in the initial apoptotic phase. Additionally, an adhesion compensatory mechanism by N-CAM180 is observed in the neuron cell body.
These results suggest a dual role of CAS in the cellular cycle control during the CY-induced apoptosis: induction of cell proliferation and stabilization of the cell-cell junctions by modulating CAMs expression.
Key words: Apoptosis; Brain; Cypermethrin; CAS. Cams.
Introduction
The CAS (cellular apoptosis susceptibility) gene is the human homolog of the yeast chromosome segregation gene CSE1 (Brinkmann et al ., 1996a; Behrens et al ., 2003). The CSE1L/CAS protein is a Ran-binding protein which acts as nuclear transport (export) factor, and simultaneously plays a role in the mitotic spindle checkpoint, which assures genomic stability during cell division. This checkpoint is frequently disturbed in neoplasias of various origins (Wellmann et al ., 2001). CAS is also implicated in the nuclear to cytoplasmic reshuffling of importin alpha, which itself is necessary for the nuclear transport of several proliferation activating proteins, transcription factors, oncogene and tumor suppressor gene products such as p53 and BRCA1 (Behrens et al ., 2003). Furthermore, CAS may have a dual function in mammalian cells, one in cell proliferation and another in apoptosis (Brinkmann et al ., 1996a).
CAS (p130CAS) was first identified as a highly tyrosine-phosphorylated 130 kilodalton protein associated with the v-Src and v-Crk-oncoproteins (Matsuda et al ., 1990; Kanner et al ., 1990, 1991; Birge et al ., 1992). Structurally, p130CAS is a scaffolding molecule that resembles a docking protein because it contains SH3 domain followed by multiple SH2 binding motifs (Sakai et al ., 1994). The SH3 domain of p130CAS is essential for focal adhesion targeting, a function that is independent of FAK binding (Nakamoto et al ., 1997). A pool of p130CAS is associated constitutively with FAK, but upon adhesion, Src kinase activity may be responsible for stabilizing the FAK- p130CAS complex (Nakamoto et al ., 1996, 1997; Polte and Hanks, 1997). The CAS proteins (p130CAS, HEF1/CAS-L and Efs/Sin), a family of docking proteins, are important components of integrin receptor signaling and have been implicated in cell-matrix adhesion, cellular migration, proliferation, transformation and apoptosis (O'Neill et al ., 2000). In humans, CAS gene is localized in the chromosome 20q13. This region is known to harbor amplifications in several cancers (Brinkmann et al ., 1996a; Wellmann et al ., 2001; Behrens et al ., 2003). Wellmann et al . (2001) found that the degree of CAS-expression correlates with the grade of tumor dedifferentiation, being a prognostic marker for neoplasms.
Normal hepatocytes reveal no CAS expression, while embryonic liver shows strong expression in all parenchymal cells. Also, bile ducts express CAS, and the interface between bile ducts and hepatocytes under conditions associated with regenerative proliferation. The localization of these CAS expressing cells is correlated with the distribution of putative liver stem-cells (Wellmann et al ., 2001). CAS is highly expressed in human tumor cell lines, in human testis and fetal liver, tissues containing actively dividing cells. Furthermore, CAS expression increases when resting human fibroblasts are induced to proliferate and decreases when they are growth-arrested. Additionally at their proliferative role, CAS appears to play an important role in both toxins and tumor necrosis factor-mediated cell death (Brinkmann et al ., 1995). For that reason, to investigate their role in the Bufo arenarum brain apoptosis induced by CY (Izaguirre et al ., 2000a, 2001a, Casco et al ., in press), the CAS expression pattern was established by immunohistochemistry.
Additionally, it is postulated that CAS could influence the cell adhesion molecules (CAMs) stabilization of cell-cell contacts (Jiang et al ., 2002). Therefore, in order to know the CAS role in the modulation of CAMs expression, and find the relation with the loss of cell-cell contacts induced by CY(Casco et al ., in press), their expression levels were quantificated by immunohistochemistry and morphometry.
Materials and Methods
Animals
Premetamorphic tadpoles of stages 28 (Gosner, 1960) control and survival of the 96-hour cypermethrin (CY)-toxicity acute test (Izaguirre et al ., 2000a, 2001a; Casco et al ., in press) were used to evaluate the CAS role in the brain apoptosis induced by CY.
The tadpoles were obtained by artificial fertilization (Izaguirre et al ., 2000b).
Bioassay
The CY toxicity test was conducted according to Casco et al . (in press). The assayed product was a commercial formulation containing 25% of CY in xylene (Sherpa, Rhône Poulenc Argentina Fomental, Buenos Aires , Argentina ). The concentrations used were 39, 156, 625, 2,500 and 10,000 µg CY/L and the mortality was registered at 24, 48, 72 and 96 h. Controls were conducted with or without vehicle (xylene) during the same periods. The lethal concentration 50 (LC-50) with confidence limits (p<0.05) was estimated by using a Probit Analysis Program (Finney, 1971). Data from control and experimental groups were analyzed by one-way analysis of variance in conjunction with LSD test (Sokal and Rohlf, 1979). Neurotoxicity was evaluated as described previously Salibián (1992).
Immunohistochemistry
Control and survival tadpoles from 24, 48, 72 and 96 hs of the CY concentration exposition (39, 156, 625, 2,500 µg CY/L) were fixed in Carnoy´s solution. The tissues were washed in phosphate buffer saline (PBS) and dehydrated in a series of increasing concentrations of ethanol, cleared by xylene and paraffin embedded (Cicarelli, San Lorenzo, Sta. Fe, Argentina). Sagittal 5 µm-thick sections were obtained with a Reichert Hn 40 microtome and placed onto 1% gelatin-coated glass slides.
Reagents. Mouse monoclonal antibodies to CAS, to E-cadherin (clone 36 mouse IgG2a) and to α - catenin (clone 5 mouse IgG1) (Transduction Labs, Lexington , KY ) were used at a 1:25 and 1:50 dilution in PBS. Rabbit polyclonal IgG to β - catenin was a gift from Dr. P. McCrea (MD Anderson Cancer Center, University of Texas ), and was used in a 1:100 dilution in PBS. Mouse monoclonal antibody (IgG1, chicken, frog) directed to a 180 kDa N-CAM polypeptide (cytoplasmic domain) was purchased from the Developmental Studies Hybridoma Bank (Dept. of Pharmacology and Molecular Sciences, Johns Hopkins Univ. School of Medicine, Baltimore , MD , and Dept. of Biological Sciences, Univ. of Iowa, Iowa City IA, USA) and used at a 1:50 dilution in PBS. Monoclonal antibody GC- 4, a mouse IgG1 to chicken N-cadherin was purchased from Sigma Chemical Co. ( St. Louis , MO ), and was used in a 1:50 dilution in PBS. Normal goat serum (Vector Laboratories, Burlingame CA , USA ) was used as negative control, diluted 1:20 in PBS. Goat anti-mouse and anti-rabbit biotinylated secondary antibodies (Vector Laboratories, Burlingame CA , USA ) were used at a 1:13 dilution. Avidin-fluorescein complex (Vectastain Elite, Vector Laboratories, Burlingame CA , USA ) was used at a 1:100 dilution. Avidin-biotin complex (ABC kit Vectastain Elite, Vector Burlingame, CA).
Cas and CAMs expression. Sagital sections at cerebral level from Bufo arenarum tadpoles were dewaxed in xylene, re-hydrated and permeabilized with 0.1% Triton X–100 (Sigma, St. Louis , MO. ) in PBS for 15 min. The sections were incubated in goat normal serum for 30 min followed by overnight incubations with the primary antibodies at 4ºC , in a wet chamber. The sections were rinsed in PBS and incubated with speciesspecific biotinylated secondary antibodies containing goat normal serum at 1:20 dilution. Sections were pretreated with 0.3% H2O2-methanol for 1 h, washed in PBS for 15 min. After, to reveal CAS expression, the sections were incubated with avidin-fluorescein complex for 30 min, rinsed two times in PBS for 5 min and mounted with Vectashield (Vector Laboratories, Burlingame CA , USA ). In contrast, to reveal CAMs expression, the sections were incubated with avidin-biotin complex for 30 min, rinsed in PBS for 5 min and color developed with 3,3'diaminobenzidine substrate (Sigma, St. Louis , MO. ). Slides were mounted with Canada balsam (Biopack , Buenos Aires , Argentina ).
Negative controls were done replacing the primary antibodies with goat normal serum diluted 1:20. Sections were examined and photographed in a BX50 Olympus microscope.
Cell adhesion molecule quantification
For cell adhesion molecule expression quantification, images of brain sagittal sections from three surviving Bufo arenarum tadpoles of both control and 96-h CY treated (2,500 µg CY/L) ( n =3) were registred using an Apogee CCD camera.
Data of immunolabelling intensity for each molecule were registred in the preestablished random areas of a squared grid applied on the right ocular of microscope. The squared grid was superposed successively through the sections brain using a X50 lens. The immunolabel intensity of the selected areas was analyzed with Image ProPlus 4.5.0.29 (Image ProPlus Software, Media Cybernetics, Inc.).
Data were expressed as mean (M) ± standard deviation (SD) and analyzed with GraphPad Instat 3.06 (GraphPad Software, San Diego , California , USA ). Mean analysis of the results were performed by the t test unpaired with and without Welch's correction conform SD and normality of the curves, or non parametric Mann-Whitney test by non normal curves. Differences were considered significant with p<0.05 values.
Results
Bioassay
High death rates (~65-70%) were observed exposing to cypermethrin at concentrations between 39 and 156 µg CY/L for 96 h (Fig. 1). There were no deaths among tadpoles treated with vehicle solution. Between 625 and 2,500 µg CY/L, the survival rate was approximately 15% at 96 h. At 24 h, practically all the tadpoles treated with 10,000 µg CY/L died (Fig. 1).

FIGURE 1. Survival curves for Bufo arenarum tadpoles exposed to cypermethrin, number of independent experiments n =3, ten larvae each.
LC50 values found at different times of exposure to CY are shown in Table 1. The LC50 values after 24 h of treatment were 6, 13 and 30 times less at 48, 72, and 96 h respectively, indicating a linear increase in toxicity with increased exposure time.

Moreover, a dose-dependent hyperactivity syndrome and abnormal behavior of the tadpoles was observed, including lateral curving of the tails, arching of the cephalic–caudal axis, twisting and sinuous writhing of the body, and circular movement activity.
CAS expression in Bufo arenarum brain
Control tadpoles of stage 28 Bufo arenarum showed CAS expression in specific cellular population of the brain (Fig. 2a), suggesting cerebral stem cells. At 24 h of the bioassay, the tadpoles treated with 39 µg CY/L showed a generalized cerebral expression (Fig. 2b). When the CY concentration increases, the CAS cellular expression also increases, which is concordant with the generalization of the programmed cellular death process (Figs. 2b-c). However, the animals treated with 2,500 µg CY/L showed a decrease in the CAS expression at the specific cellular groups (Fig. 2d).
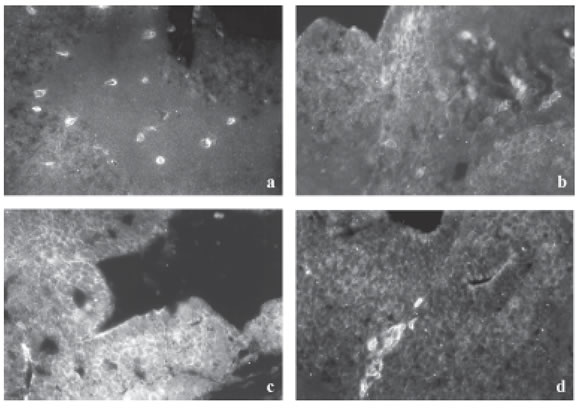
FIGURE 2. CAS expression in the brain of stage 28 Bufo arenarum tadpoles (a) control and treated with CY during 24 h (b-d) (133X). Cas expression increases from 39 µg CY/L (b) to 156 mg CY/L (c), correlated with the increase of cellular apoptosis. However, from 2,500 µg CY/L (d) Cas decrease exhibing high immunolabel on specific cellular groups.
At 48 h of CY treatment, the same behavior is verified in the CAS expression than that observed at 24 h (Figs. 3a-c). In contrast, at 72 h only the 39 µg CY/L concentration displays high levels of CAS, decreasing suddenly its expression at higher concentrations (Fig. 3d), whereas at 96 h no signal was detected for all concentrations used.

FIGURE 3. CAS expression in the brain of stage 28 Bufo arenarum tadpoles treated with CY during 48 h (a-c) and 72 h (d) (133X). The time increase (48 h) of the CY treatment produces a Cas cellular expression decrease with the increase of the CY dosis from 39 µg CY/L (a) to 156 µg CY/L (b) and to 2,500 µg CY/L (c). This decrease is pronounced from 72 h treatment (d) where the tadpoles treated with 39 µg CY/L exhibit high immunolabel on specific cellular groups, similar that occurs with high CY concentration at 24 h. At 96 h no CAS signal was detected for none CY doses.
In all cases, CAS was expressed in the cellular cytoplasm, and was not detected at nuclear level. The CAS regional expression does not exhibits a specific pattern.
CAMs expression in Bufo arenarum brain
The control tadpole central nervous system exhibits N-CAM180 and N-cadherin expression in neuronal soma and axons. The expression of N-CAM180 in neurons was significantly altered by CY treatment. In contrast to the relatively homogeneous expression of NCAM180 observed in the neurons of control tadpoles, CY-treated tadpoles exhibit a significant increase of NCAM180 in the neuronal cell body (116.5973 vs. 123.5720, p < 0.005) (Figs. 4a-b and 5) and a significant decrease in the axons regions (144.4598 vs. 141.3270, p < 0.005) (Figs. 4a-b and 5).
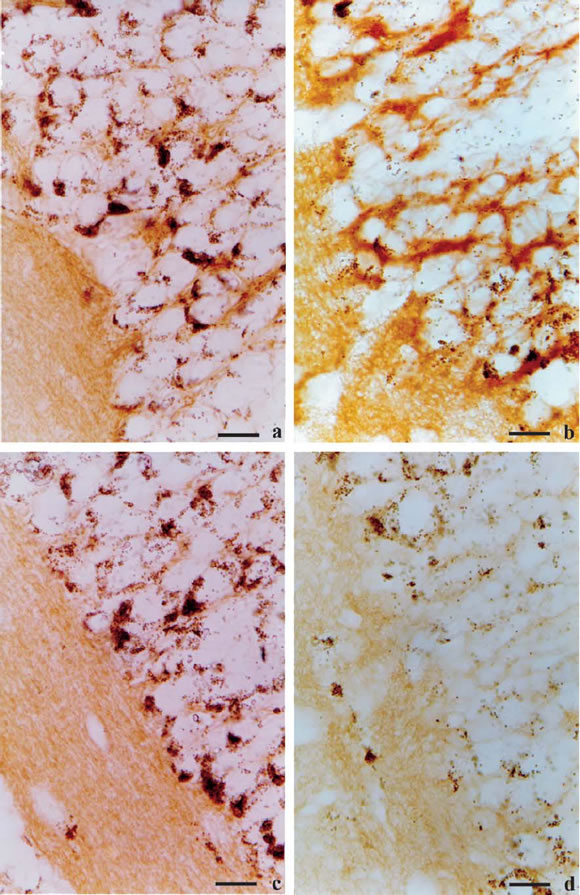
FIGURE 4. Sagittal sections of control and 2,500 µgCY/L-treated at 96 h Bufo arenarum tadpoles at central nervous system level. (a) N-CAM180 expression in control tadpoles, X333 and (b) CY-treated tadpoles, X333. (c) β-catenin expression in control tadpoles, X333 and (d) CY-treated tadpoles, X333.

FIGURE 5. N-CAM180 expression on the neuronal cell bodies (NCB) and axons of Bufo arenarum tadpole brains treated with 2,500 µg CY/L at 96 h. NCB CY-treated vs. control: * P <0.005; Axons CY-treated vs . control: * P <0.005.
A discernible expression of β-catenin was detected on immature neurons and choroid plexus in the central nervous system (Fig. 4c). In CY-treated animals, β-catenin showed a significantly decreased expression at axons compared with vehicle-treated controls (156.4825 vs. 165.3559, p < 0.005) (Figs. 4c-d and 6). However, neuronal cell body, exhibit not significantly differences (136.3876 vs. 130.7324) (Figs. 4c-d and 6). The levels of α-catenin remained unchanged after cypermethrin treatment (not shown).
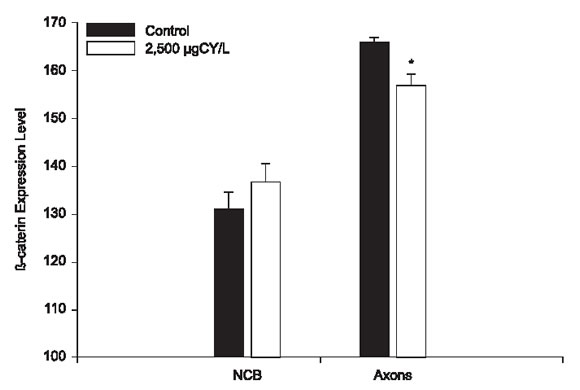
FIGURE 6. β-catenin expression on the neuronal cell bodies and axons of Bufo arenarum tadpole brains treated with 2,500 µg CY/L at 96 h. NCB Cytreated vs. control: no significant ; Axons CY-treated vs . control: * P <0.005.
Corroborating our previous studies of normal larval development (Izaguirre et al ., 2001b), E-cadherin was detected only in the peripheral nervous system level and did not exhibit changes of its expression pattern in CY-treated tadpoles (not shown).
Discussion
The CAS gene is highly conserved in eukaryotes, it consists of 25 exons. The mRNA homologous over its entire length to the yeast homologue CSE1 is the predominant transcript in proliferating tissues. Additional mRNAs are generated by alternative splicing in tissuespecific manner. An extended 3'-end is found in fetal and adult brain. mRNA containing the 5'-end of CAS up to position 690 and an alternative 3'-end is expressed in trachea and encodes a truncated Ran-binding domain. Fetal liver expresses mRNA with deletions of a central portion of CAS and additional sequences encoded by the last intron. SW480 colon cancer cells express another approximately 1500-base mRNA. It is known that there is a correlation of CAS transcripts and CAS protein in different tissues (Brinkmann et al ., 1999). Thus, CAS isoforms may control nuclear transport of tissuespecific proteins. Therefore, hCSE1/CAS can play a role in several and oppose processed as the cellular proliferation and apoptosis (Brinkmann et al ., 1996a; Behrens et al., 2003).
In order to determine if the protein CAS act as an anti-apoptotic factor in the Bufo arenarum cerebral apoptosis induced by the CY toxin, stimulating cellular proliferation, the CAS expression pattern was established by immunohistochemistry techniques.
Cypermethrin (RS)-alpha-cyano-3-phenoxybenzyl (1RS)-cis-, trans-3- (2, 2, -dichlorovinyl)-2, 2- dimethylcyclopropane carboxylate) is a highly active synthetic pyrethroid type II. These are among the most active insecticides and at concentrations as low as 0.02 to 0.05% (5 to 200 g/L ha) can be as efficient as organophosphorous compounds (Kaloyanova et al ., 1991).
Pyrethroids are characterized by strong, broad-spectrum insecticidal activity, based on their neurotoxicity (Cole and Casida, 1983; Kaloyanova et al., 1991; Salibián, 1992; Izaguirre et al., 2000a; 2001a; Casco et al., in press). They act on the axons of the peripheral and central nervous system by interacting with sodium channels gating mechanisms. Synthetic pyrethroids induce delaying in closing of sodium channels (Kaloyanova et al ., 1991) producing alterations in the ion conductance of nerve cell membranes, which may be one of the mechanisms of pyrethroid toxicity (Salibián and Marazzo, 1995). This in turn results in increased transmembrane sodium influx and inhibition of ion-dependent ATPases in the nervous systems of insects, squids and toads (Berlin et al., 1984). One by changes in the flow of K+ and Na+ are known to play a critical role in the activation of neuronal apoptosis. Our studies have demonstrated that lower doses than those used in routine applications can cause massive apoptosis in the central nervous system of anuran larvae (Izaguirre et al., 2000a, 2001a; Casco et al., in press).
The present work demonstrate that during the first hours of the CY intoxication, the CAS expression is increased in the brain when are used low doses of CY (39 - 156 µg CY/L). These results suggest that the survival brain cells increase CAS synthesis and /or brain stem cells could proliferate opposing the apoptosis induced by cypermethrin. Recently, Wellmann et al . (2001) have found that the localization of CAS expressing cells is correlated with the distribution of putative stem-cells. However, when the CY doses increase (up to 2,500 µg CY/L) or the CY exposition times reach at 96 h, CAS expression became restricted to specific cellular groups, like in the normal brain. Present results allow us to speculate that, under these conditions, CAS expression may be unable to act as a survival signal.
Scherf et al . (1996) found that CAS is present in the cytoplasm of proliferating cells at levels between 2 x 105 and 1 x 106 molecules per cell. The intracellular distribution of CAS resembles that of tubulin. Thus, in interphase cells, CAS shows microtubule-like patterns and in mitotic cells it labels the mitotic spindle. However, CAS appears to be associated with, but not to be an integral part of microtubules. Elevated amounts of CAS are found in proliferating cells such as testicular spermatogonia and cells in the basal layer of the colon. CAS was also concentrated in the respiratory epithelium of the trachea and in axons and Purkinje cells in the cerebellum. These cells contain many microtubules. The cellular location of CAS is consistent with an important role in cell division as well as in ciliary movement and vesicular transport (Scherf et al ., 1996).
In the other hand, it has been reported that the expression of CAS is up regulated in a variety of human tumor cells, and such expression correlates with the development of tumors, and with the susceptibility of tumor cells to IFN-gamma-induced apoptosis. The IRF-1 transcriptional factor mediates IFN-gamma-induced CAS expression. CAS over expression via IFN-gamma enhances the CPP32 cellular expression and apoptosis. Because of CPP32 is regarded as one of the central apoptosis executioner molecules, it is possible that tumor cells highly expressing CAS may be more susceptible to apoptosis induced by reagents that are capable of inducing CAS expression. Thus, CAS may be a target for the elimination of tumors (Jiang et al ., 2001). Therefore, CAS is not only up regulated in proliferate cells but also in apoptotic cells induced by IFN-gamma. However, our previous results (Casco et al ., in press) suggest that the CAS expression increases is doubt at the protective factor to the apoptosis and non for cellular increase of apoptosis susceptibility, as is demonstrated for the CY dose- and time-dependent apoptosis increase, and the Cy doses- and times-dependent CAS expression decrease.
These results show that a single molecule can participate in proliferation and apoptosis through of the CAS expression increase. In support at these results, Brinkmann et al. (1996b), working with an antisense cDNA fragment homologous to the yeast chromosome segregation gene CSE1, found that those reduces the intracellular content of the human CSE1 homologue CAS protein. CAS reduction confers resistance not only to the ADP-ribosylating toxins PE and DT, but also to tumor necrosis factor alpha and beta. The resistance was observed as reduced apoptosis. CAS antisense did not affect the cell death induced by staurosporine, cycloheximide, or etoposide. The observation that CAS antisense can interfere with apoptosis mediated by TNF and ADP-ribosylating toxins suggests that CAS may play a role in selected pathways of apoptosis.
It is known that SRC family kinases play essential roles in a variety of cellular functions, including proliferation, survival, differentiation, and apoptosis. The activities of these kinases are regulated by intramolecular interactions and by heterologous binding partners that modulate the transition between active and inactive structural conformations. p130(CAS) binds directly to both the SH2 and SH3 domains of c-SRC and therefore has the potential to structurally alter and activate this kinase. The c-SRC activation induces c-SRC-dependent tyrosine phosphorylation of multiple endogenous cellular proteins, acting as an adaptor protein. These events suggest that CAS plays an important role in regulating specific signaling pathways governing cell growth and/or survival, in part through its ability to interact with and modulate the activity of c-SRC (Burnham et al ., 2000). Therefore, through its ability to undergo rapid changes in phosphorylation, subcellular localization and association with heterologous proteins, CAS may spatially and temporally regulate the function of its binding partners. Numerous proteins have been identified that bind to CAS in vitro and/or in vivo , but in only a few cases there is an understanding of how CAS may function in these protein complexes. To date, CASCrk and CAS-Src complexes have been most frequently implicated in CAS function, particularly in regards to processes involving regulation of the actin cytoskeleton and proliferation.
It is well known that in normal cells, the p130CAS-associated tyrosine kinase activities are anchorage-dependent. Thus, cell detachment triggers rapid dephosphorylation of p130CAS in the anoikis-sensitive normal epithelial cells. FAK and Src are p130CAS-associated tyrosine kinases, which tyrosine phosphorylating mechanisms are altered in the tumor cells. Inhibition of Src specifically abolished phosphorylation of p130CAS and induced anoikis. Furthermore, over expression of dominant-negative forms of p130CAS also induced apoptosis. Taken together, these data suggest that p130CAS mediates a cell survival signal from cell-matrix interaction. Alterations in tumor cells that lead to constitutive phosphorylation of p130CAS can prevent cells from anoikis; hence contribute to tumor cell anchorage independence and metastasis (Wei et al ., 2002). Furthermore, Jiang et al . (2002) found that the interaction of CAS with E-cadherin, a cell-cell adhesion molecule, enhances the formation of E-cadherin/beta-catenin cell-cell adhesive complex. Their results indicate that CAS cooperates with E-cadherin and plays a role in the establishment of cell-cell interactions and epithelial cell polarity.
Our results suggest that exists a relation between neuronal apoptosis and survival and cell adhesion mediated by CAMs. Cell adhesion is a multifactorial mechanism that depends on the expression of tissuespecific isoforms of cell adhesion proteins, surface density of individual molecules and regulatory factors (Edelman, 1988). N-cadherin and N-CAM both play a key role in the central nervous system morphogenesis and function in vertebrates (Rutishauser, 1984; Hatta and Takeichi, 1986; Cunningham et al ., 1987; Rutishauser and Jessell, 1988; Neugebauer et al., 1988; Matsunaga et al., 1988; Drazba and Lemmon, 1990; Bixby and Zhang, 1990; Takeichi et al., 1991; Doherty et al., 1991; Paz et al ., 1995; Izaguirre et al., 2000b). Catenins are part of a sub-membranous protein network by which cadherins are connected to other integral membrane proteins (McNeill et al., 1990). Catenins regulate the extracellular adhesive properties of cadherins as well as their interaction with the cytoskeleton (Kemler et al ., 1990). Catenins also mediate signals for gene expression during development and tissue morphogenesis (Matsuyoshi et al ., 1992; Rubinfeld et al., 1993; Su et al., 1993; Hamaguchi et al ., 1993; Behrens et al., 1993; Shibamoto et al., 1994; Hoschuetzky et al., 1994). In this study, we did not detect changes in the expression pattern of N-cadherin, E-cadherin and α-catenin due to CY treatment. These results suggest that the loss of cellular contacts of Cyinduced apoptotic cells in the central nervous system may not completely depend on down regulation of cell adhesion molecules expression but possibly on loss of functionality. Additionally, we verified that NCAM180 increased in neuronal cell body, likely as a compensatory mechanism against the loss of cellular contact and also as a protective factor inhibiting apoptosis. It is known that neural cell adhesion molecule plays a pivotal role in neural development, regeneration, and plasticity. N-CAM mediates cell adhesion and subsequent signal transduction through N-CAM - N-CAM binding. Pedersen et al . (2004) demonstrated that a small peptide mimicking homophilic N-CAM interaction is capable of inducing differentiation in several neuronal cell types and inhibiting apoptosis in cerebellar granule neurons. However, NCAM expression decreased in the axons. In support of this result it is known that in a mature neuron, polarized targeting of proteins from the cell body to the axonal and dendritic processes is essential for its proper function, especially, for the maintenance of synaptic function. Any disruption of this protein distribution can lead to a disturbance in synaptic transmission and produce neurodegeneration.
The expression of β-catenin expression was also significantly decreased in axons suggesting catenin cleavage during the apoptosis. In agreement with these results, other studies have showed loss of cell-cell interactions and catenin cleavage by caspases during apoptosis (Brancolini et al ., 1998). The caspase family of cystein proteases plays a key role during the execution phase of the apoptotic program. These proteolytic enzymes, once activated, cleave cellular proteins which are important for the maintenance of cell integrity. Multiple proteolytic events regulate the dismantling of the cell-cell junctional complexes during apoptosis (Brancolini et al ., 1998). However, other studies demonstrate that the proteolysis and changes of many cytoskeletal proteins associated with apoptosis precede degradation of catenins and cadherins by several hours (Schmeiser and Grand, 1999). Apparently, caspase-mediated degradation of cytoskeletal components, or disruption of adherens junction protein-protein interactions are not required for the morphological changes that occur during the apoptosis process (Schmeiser and Grand, 1999). Furthermore, it is known that in Alzheimer's disease phospho-beta-catenin accumulates in cytoplasmic inclusions that coalesce into granulovacuolar degeneration bodies and colocalize with gamma-tubulin and vimentin. These aggregates that might result from impaired proteasome function were associated with apoptotic cell death and with activation of caspase-3, c-Jun-N-terminal kinases, and c-Jun (Ghanevati and Miller, 2005).
The present work demonstrates the key role of CAS in the apoptotic process induced by CY, but additional experiments will be necessary to establish the intricate signaling mechanisms involved. Additionally, it has been demonstrated that whereas some cell adhesion molecules (CAMs) increase during apoptosis others are unaltered or decreased, suggesting different roles of adhesion proteins in this process. The loss of cellular contacts that is produced can occur without degradation of N-cadherin and α-catenin at least in the initial phases of the apoptotic mechanism or with decrease of β-catenin suggesting their cleavage. In contrast, NCAM180 shows selective cell-cell adhesion mechanisms. Increased N-CAM180 in the neuronal body may reflect activation of anti- apoptotic mechanisms, whereas decrease in the axons may be the result of protein cleavage during apoptosis, or an effect of redistribution of molecules into plasmatic membrane from axons to soma. These results demonstrate the alterations on cell-cell adhesion induced by CY. Low doses and short time of CY treatment are associated with CAS expression increase and cell-cell adhesion molecules expression control.
These results suggest a dual role of CAS in the cellular cycle control during the CY-induced apoptosis, by inducing cell proliferation and by stabilization of the cell-cell junctions by modulating cell-cell adhesion molecule expression.
Acknowledgements
This work was supported by a grant from SCYTFRH-UNER, PID 6053-1 (to VHC).
References
1. Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin / β-catenin complex in cells transformed with a temperature-sensitive v-src gene. J Cell Biol. 120: 757-766. [ Links ]
2. Behrens P, Brinkmann U, Wellmann A (2003). CSE1L/CAS: its role in proliferation and apoptosis. Apoptosis. 8: 39-44. [ Links ]
3. Berlin JR, Akera T, Brody TM, Matsumura F (1984). The ionotropic effects of a synthetic pyrethroid decamethrin on isolated guinea pig atrial muscle. Eur J Pharmacol. 98: 313-322. [ Links ]
4. Birge RB, Fajardo JE, Mayer BJ, Hanafusa H (1992). Tyrosinephosphorylated epidermal growth factor receptor and cellular p130 provide high affinity binding substrates to analyze Crk-phosphotyrosine-dependent interactions in vitro . J Biol Chem. 267: 10588-10595. [ Links ]
5. Bixby JL, Zhang R (1990). Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 110: 1253-1260. [ Links ]
6. Brancolini C, Sgorbissa A, Schneider C (1998). Proteolytic processing of the adherens junctions components beta-catenin and gamma-catenin/plakoglobin during apoptosis. Cell Death Differ. 5: 1042-1050. [ Links ]
7. Brinkmann U, Brinkmann E, Gallo M, Pastan I (1995). Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc Natl Acad Sci U S A. 92: 10427-10431. [ Links ]
8. Brinkmann U, Gallo M, Polymeropoulos MH, Pastan I (1996a). The human CAS (cellular apoptosis susceptibility) gene mapping on chromosome 20q13 is amplified in BT474 breast cancer cells and part of aberrant chromosomes in breast and colon cancer cell lines. Genome Res. 6: 187-194. [ Links ]
9. Brinkmann U, Brinkmann E, Gallo M, Scherf U, Pastan I (1996b). Role of CAS, a human homologue to the yeast chromosome segregation gene CSE1, in toxin and tumor necrosis factor mediated apoptosis. Biochemistry. 35: 6891-6899. [ Links ]
10. Brinkmann U, Brinkmann E, Bera TK, Wellmann A, Pastan I (1999). Tissue-specific alternative splicing of the CSE1L/CAS (cellular apoptosis susceptibility) gene. Genomics. 58: 41-49. [ Links ]
11. Burnham MR, Bruce-Staskal PJ, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH (2000). Regulation of c-SRC activity and function by the adapter protein CAS. Mol Cell Biol. 20: 5865-5878. [ Links ]
12. Casco VH, Izaguirre MF, Marín L, Vergara MN, Lajmanovich, RC, Peltzer P, Peralta Soler A (in press). Apoptotic cell death in the central nervous system of Bufo arenarum tadpoles induced by cypermethrin. Cell Biol Toxicol. [ Links ]
13. Cole LM, Casida JE (1983). Pyrethroid Toxicology in the Frog. Pesticide Biochem Phys. 20: 217-224. [ Links ]
14. Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM (1987). Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 236:799-806. [ Links ]
15. Doherty P, Rowett LH, Moore SE, Mann DA, Walsh SW (1991). Neurite outgrowth in response to transfected N-CAM and Ncadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 6: 245-258. [ Links ]
16. Drazba J, Lemmon V (1990). The role of cell adhesion molecules in neurite outgrowth on Müller cells. Dev Biol. 138: 82-93. [ Links ]
17. Edelman GM (1988). Morphoregulatory molecules. Biochemistry. 27: 3533-3543. [ Links ]
18. Finney DJ (1971). Probit analysis. 3rd ed. Cambridge University Press, New York , pp. 668. [ Links ]
19. Ghanevati M, Miller CA (2005). Phospho-beta-catenin accumulation in Alzheimer's disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci. 25:79-94. [ Links ]
20. Gosner KL (1960). A simplified table for staging anuran embryos and larvae, with notes on identification. Herpetológica. 16: 183-190. [ Links ]
21. Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y (1993). p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 12: 307-314. [ Links ]
22. Hatta K, Takeichi M (1986). Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 320: 447-449. [ Links ]
23. Hoschuetzky H, Aberle H, Kemler R (1994). β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 127: 1375-1380. [ Links ]
24. Izaguirre MF, Lajmanovich RC, Peltzer PM, Peralta Soler A, Casco VH (2000a). Cypermethrin- Induced Apoptosis in the Telencephalon of Physalaemus biligonigerus Tadpoles (Anura:Leptodactylidae). Bull Environ Contam Toxicol. 65: 501-507. [ Links ]
25. Izaguirre MF, Peralta Soler A, Casco VH (2000b). Expression of NCAM-180 and N-cadherin during development in two southamerican anuran species ( Bufo arenarum and Hyla nana ). Eur J Histochem. 44: 407-418. [ Links ]
26. Izaguirre MF, Lajmanovich RC, Peltzer PM, Peralta Soler A, Casco VH (2001a). Induction of cell Death by the Synthetic Pyrethroid Insecticide Cypermethrin in the Developing Brain of Physalaemus biligonigerus Tadpoles from Argentina . Froglog. 43-3. [ Links ]
27. Izaguirre MF, Adur JF, Peralta Soler A, Casco VH (2001b). Alterations induced by E-Cadherin and β-catenin antibodies during the development of Bufo arenarum (Anura – Bufonidae). Histol Hitopathol. 16: 1097-1106. [ Links ]
28. Jiang MC, Lin TL, Lee TL, Huang HT, Lin CL, Liao CF (2001). IRF-1-mediated CAS expression enhances interferon-gammainduced apoptosis of HT-29 colon adenocarcinoma cells. Mol Cell Biol Res Commun. 4: 353-358. [ Links ]
29. Jiang MC, Liao CF, Tai CC (2002). CAS/CSE 1 stimulates Ecadhrin-dependent cell polarity in HT-29 human colon epithelial cells. Biochem Biophys Res Commun. 294: 900-905. [ Links ]
30. Kaloyanova F, Batawi E, Mostafa A (1991). Human toxicology of pesticides. CRC, Press, Inc Boca Ratón, 208pp. [ Links ]
31. Kanner SB, Reynolds AB, Vines RR, Parsons JT (1990). Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kynases. Proc Natl Acad Sci USA 87: 3328-3332. [ Links ]
32. Kanner SB, Reynolds AB, Wang HC, Vines RR, Parsons JT (1991). The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 10: 1689-1698. [ Links ]
33. Kemler R, Gossler A, Mansouri A, Vestweber D (1990). The cell adhesion molecule uvomorulin. In: Morphoregulatory molecules. Edelman GM, Cunningham BA, Thiery JP, eds. Inc New York : John Wiley and Sons, pp. 41-56. [ Links ]
34. Matsuda M, Mayer BJ, Fukui Y, Hanafusa H (1990). Binding of transforming protein, p47gag-crk, to a broad range of phosphotyrosinecontaining proteins. Science. 248: 1537-1539. [ Links ]
35. Matsunaga M, Hatta K, Nagafuchi A, Takeichi M (1988). Guidance of optic nerve fibers by N-cadherin adhesion molecules. Nature. 334: 62-64. [ Links ]
36. Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M (1992). Cadherin-mediated cell-cell adhesion is perturbed by src-tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 118: 703-714. [ Links ]
37. McNeill H, Ozawa M, Kemler R, Nelson WJ (1990). Novel function of the cell adhesion molecule E-cadherin as an inducer of cell surface polarity. Cell. 62: 309-316. [ Links ]
38. Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H (1996). Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J Biol Chem. 271: 8959-8965. [ Links ]
39. Nakamoto T, Sakai R, Honda H, Ogawa S, Ueno H, Suzuki T, Aizawa S-I, Yazaki Y, Hirai H (1997). Requirements for localization of p130Cas to focal adhesions. Mol Cell Biol. 17: 3884-3897. [ Links ]
40. Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF (1988). Ncadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro . J Cell Biol. 107: 1177-1187. [ Links ]
41. O'Neill GM, Fashena SJ, Golemis EA, Scherf U, Kalab P, Dasso M, Pastan I, Brinkmann U (2000). Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 10:111-119. [ Links ]
42. Paz DA, Alonso DG, Pisanó A, Casco VH, Knudsen K, Peralta Soler A (1995). Expression of isoforms of the neural cell adhesion molecule (NCAM) and polysialic acid during the development of the Bufo arenarum olfactory system. Int J Dev Biol. 39: 1005-1013. [ Links ]
43. Pedersen MV, Kohler LB, Ditlevsen DK, Li S, Berezin V, Bock E (2004). Neuritogenic and survival-promoting effects of the P2 peptide derived from a homophilic binding site in the neural cell adhesion molecule. J Neurosci Res. 75: 55-65. [ Links ]
44. Polte TR, Hanks SK (1997). Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130(Cas)) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK-proline-rich motifs. J Biol Chem. 272: 5501-5509. [ Links ]
45. Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P (1993). Association of the APC gene product with b-catenin. Science. 262: 1731-1734. [ Links ]
46. Rutishauser U (1984). Developmental biology of a neural cell adhesion molecule. Nature. 310: 549-554. [ Links ]
47. Rutishauser U, Jessell TM (1988). Cell adhesion molecules in vertebrate neural development. Physiol Rev. 68: 819-857. [ Links ]
48. Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H (1994). A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 13: 3748-3756. [ Links ]
49. Salibián A (1992). Effects of deltametrhin on the south american toad (Bufo arenarum ). Bul Eviron Contam Toxicol. 48: 616-621. [ Links ]
50. Salibián A, Marazzo J (1995). Studies on the Effects of Deltamethrin on Sodium Net Transport Through the in vivo Amphibian Skin. Biomed Environ Sci 8: 164-168. [ Links ]
51. Scherf U, Pastan I, Willingham MC, Brinkmann U (1996). The human CAS protein which is homologous to the CSE1 yeast chromosome segregation gene product is associated with microtubules and mitotic spindle. Proc Natl Acad Sci U S A. 93: 2670-2674. [ Links ]
52. Schmeiser K, Grand RJ (1999). The fate of E- and P-cadherin during early stages of apoptosis. Cell Death Differ. 6: 377-386. [ Links ]
53. Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F (1994). Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1: 295-305. [ Links ]
54. Sokal RR, Rohlf FJ (1979). Biometría. Ediciones Blume, Madrid 830pp. [ Links ]
55. Su LK, Vogelstein B, Kinzler KW (1993). Association of the APC tumor suppressor protein with catenins. Science. 262: 1734-1737. [ Links ]
56. Takeichi M, Inuzuka H, Shimamura K, Fujimori T, Nagafuchi A (1991). Cadherin subclasses: differential expression and their roles in neural morphogenesis. Cold Spring Harb Symp Quant Biol. 55: 319-325. [ Links ]
57. Wei L, Yang Y, Zhang X, Yu Q (2002). Anchorage-independent phosphorylation of p130(Cas) protects lung adenocarcinoma cells from anoikis. J Cell Biochem. 87: 439-449. [ Links ]
58. Wellmann A, Flemming P, Behrens P, Wuppermann K, Lang H, Oldhafer K, Pastan I, Brinkmann U (2001). High expression of the proliferation and apoptosis associated CSE1L/CAS gene in hepatitis and liver neoplasms: correlation with tumor progression. Int J Mol Med. 7: 489-494. [ Links ]
Received on December 27, 2005.
Accepted on March 30, 2006.














