Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.30 no.3 Mendoza Ago./Dec. 2006
Chronic stress effects on the apoptotic index of the adrenal cortex of pregnant rats
Andrea Bozzo*, Carlos Alberto Soñez*, María Teresa Mugnaini*, Isabel Cecilia Pastorino*, Alicia Nélida Rolando*, María Cristina Romanini*, and Héctor Fernando Gauna**.
* Biología Celular y Embriología General, Facultad de Agronomía y Veterinaria, Universidad Nacional de Río Cuarto, Argentina.
** Fisología Animal, Facultad de Ciencias Exactas, Físico-Químicas y Naturales, Universidad Nacional de Río Cuarto, Argentina.
Address correspondence to: Ms.Sc. Andrea A. Bozzo. Cátedra de Biología Celular y Embriología General. Facultad de Agronomía y Veterinaria. Universidad Nacional de Río Cuarto, (CP. X5804BYA) Róo Cuarto, Córdoba, ARGENTINA. Fax: (+54-358) 4680280. E-mail: abozzo@ayv.unrc.edu.ar
ABSTRACT: Chronic stress by immobilization during gestation can alter several mechanisms that maintain homeostasis in adrenal gland. The aim of this work was to quantify the apoptotic index of adrenal cortex during mid-pregnancy and to prove cytological characteristics by electron microscopy. The apoptotic index did not present significant differences between the adrenal cortex areas of control and experimental rats in any of the three ages studied. The day of gestation influenced significantly on the apoptotic index in both groups. This index increased as gestation progressed. It may be concluded that chronic stress by immobilization might induce the increase of apoptotic index in adrenal cortex as gestation progresses which might be related variations of plasmatic corticosterone and prolactin, and to the decrease of specific growth factors. On the other hand, it might be concluded that each zone of adrenal cortex behaves independently in regards to apoptosis and cellular proliferation via paracrine and/or autocrine regulatory mechanisms without being affected by other zones.
Key words: Chronic stress; Apoptosis; Adrenal cortex; Pregnancy.
Introduction
There are some works using " in vivo" pattern under the conditions of chronic stress by immobilization (IMO) but its effect on the apoptosis of pregnant rats adrenal glands has not been demonstrated yet. Only a few reports have described apoptotic process on adrenal glands under normal conditions in non pregnant animals (Blanco et al., 2001). Using the same experimental pattern of chronic stress, we demonstrated that some variations in plasmatic levels of prolactin (PRL) and maternal corticosterone (CORT) are produced (Soñez et al., 1996; Romanini et al., 1999).
The adaptation of adrenal gland to the organism demands is functional and structurally regulated. Cell death by apoptosis plays an essential role on adrenocortical remodeling during embrionary period and postnatal development (Yeastin, 1986; Ducsay et al., 1991).
The adrenal gland, as an organ responding to stress, is subject to dynamic structural changes, including both proliferation as well as cell death. A balance between these two processes is essential for integrity and functionality of this organ. Regulatory systems of the organism, and the autocrine and paracrine local networks determine the cell cycle balance (Wolkersdorfer and Bornstein, 1998).
In rat adrenal cortex, the cells composing each zone are continuously renewed by migration of new cells derivated from progenitor cells that differentiate and die. Thus, apoptosis regulation in adrenal gland may be essential for the definition of the functional zonal architecture (Mitani et al., 1994).
In this context, the current theory of adrenocortical zonalization should be re-evaluated to achieve a new interpretation about regulatory mechanisms on apoptosis and cell proliferation in accordance with the contribution of the new findings reported.
In 1998, Wolkersdorfer and Bornstein proposed three theories to explain the zonalization of adrenal cortex. The migration theory describes cell proliferation in the cortex outer part, their migration and differentiation from glomerular zone to fascicular zone and from this to reticular zone where the cells degenerate and die. On the other hand, the transformation theory proposes two transformation directions considering the replacement of zonal tissue by proliferant cells. These transformation zones are placed between the glomerular zone and the fascicular zone from which migration takes place in two opposite directions: on one side towards the medulla and on the other towards the capsule. Finally, the zonal theory proposes an equal proliferation in the three zones. Since the apoptosis also takes place in the three cortex zones, each zone could be locally regulated without being affected by other zones. All these theories intend to explain the phenomenon of maintaining structural homeostasis and the relationship between proliferation and programmed cell death (Wolkersdorfer and Bornstein, 1998).
The aim of this work was to characterize apoptosis in adrenal glands of normal pregnant rats and to prove the effects of chronic stress by intermittent immobilization during the second half of gestation.
Materials and Methods
Materials
Wistar primipara rats aged three months were used. They were maintained under controlled laboratory conditions with food and water ad libitum, contant photoperiod (12/12 h. light/darkness), temperature 20º ± 2ºC and room humidity. (Laboratory installations were adequate to disposition 6344/96 of the Administración Nacional de Medicamentos, Alimentos y Tecnología Médica, Argentina). The Conclusions and Recommendation on the Reduction, Refinement and Replacement of Laboratory Animals Procedure of Declaration of Bologna (Russell and Burch, 1959) were followed for animal experimentation. All experiments were conducted according to the principles and procedures of the NIH Guide for the Care and Use Laboratory Animals (NIH publication nº 85-23, revised 1985; http:// www.nih.gov/sigs/bioethics).
Females were cycled with colpocytograms and they were mated during the proestrous with a same strain male. The zero pregnant day was stated at 24 hours of verifying spermatozoids in vaginal fluid. Pregnant rats were separated into two groups: control (CR) and experimental (ER). From the fourth gestation day the ER group was subjected to IMO stress sessions on boards of 45 minutes each, every other day, until the day previous to sacrifice according to the method described by Michajlovskij et al. (1988). CR and ER were sacrificed by decapitation at 12, 17 and 21 days of gestation. Five (5) rats per gestation age for each group were obtained (n=30 per group). The adrenal glands of each pregnant rat from CR and ER groups were extracted previous fixation in 4% formaldehyde in phosphate buffer. Adrenal glands were processed according to the conventional paraffine histological technique. 5mm thickness serial sections were obtained from adrenal cortex and fixed with VectabondÒ adhesive (Vector Laboratories, Inc, Burlingame, USA). Alternate serial sections were stained using H&E. The identification of glomerular, fascicular and reticular zones of the adrenal cortex was performed considering their classical histological characteristics.
TUNEL Immunocytochemical Technique
Twelve alternate sections were processed from each animal of CR and ER groups at all studied ages. The hydrated sections were incubated with K-proteinase during 8 - 10 min in wet chamber. Endogen peroxidase blocking was done with 3% hydrogen peroxide following the protocol provided by the Apoptag Plus "in situ Apoptosis Peroxidase" (Oncor, USA) kit. Negative control did not include the terminal transferase desoxinucleotidyl transferase enzyme (TdT); in the positive control, post-lactation mammary gland was used. Normal nuclei were identified using 1% methyl green nuclear contrast coloration.
Electron Microscopy
Apoptotic phenomenon was proved by conventional electron microscopy (fixation with 4% buffered glutaraldehyde, post-fixation with 1% OsO4, inclusion in EPON 812 and contrast with uranile acetate and plumb citrate). It was observed using an Jeol JEM 1200 EX II.
Images Stereological Analysis
Quantification of apoptotic and normal nuclei was performed using the System KS-300 v. 3.0 (Kontron/ Zeiss) and the elaboration of an "ad-hoc" macro program. Seven to ten images of each cortical zone were digitalized from each histological section totalizing 750 CR and ER images. A Zeiss Axioscope microscope with a built in 3.2 Mpx Sony digital camera was used.
Statistical Analysis
The apoptotic index (A.I.) was obtained from the ratio between apoptotic and normal nuclei multiplied by 100. Data analysis was conduted using monofactorial ANOVA, trifactorial ANOVA and the Kruskal-Wallis tests. Those values over 5% were considered to be significant. All the analyses were performed using the S.P.S.S. V.9.0 software (S.P.S.S. Inc., 444 N. Michigan Avenue, Chicago, IL 60611).
Results
Different zones of the adrenal cortex with apoptotic and normal nuclei are shown in Fig. 1 use. Apoptotic nuclei were present in all adrenal cortex zones.
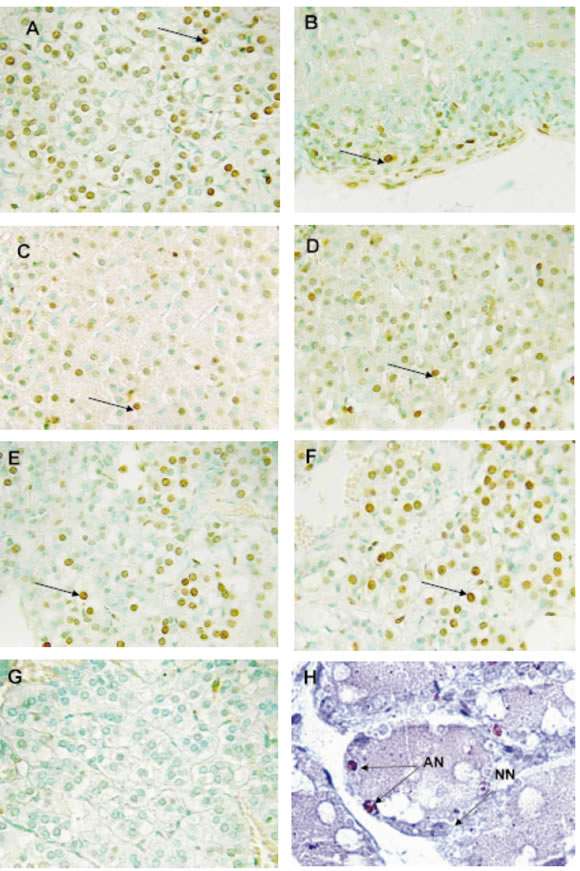
FIGURE 1. Histological sections of the adrenal cortex zones from control (A, C, E) and stressed rats (B, D, F) during middle pregnancy (12th days: A, B; 17th days: C, D), and late pregnancy (21th days: E, F) stained by using the methyl green -TUNEL technique. Arrows indicate positive TUNEL nuclei. Glomerular zone: A, B; fascicular zone: C, D; reticular zone: E, F, G. The negative control of TUNEL technique does not show any apoptotic signs. H: Post-lactation mammary gland use as positive control of TUNEL technique. Arrows indicate apoptotic nuclei (AN); NN: normal nucleus (X 650).
Under electron microscope, apoptotic signs were observed in individual cells such as chromatin fragmentation, morphology and nuclear membrane integrity loss, cytoplasm condensation and general disorganization (Figs. 2A, 2C). It was also observed apoptotic bodies phagocyted by glomerular zone cell with different organelles and nuclear rests under auto-phagocytosis process maintaining plasmatic membrane (Fig. 2B), and degraded apoptotic body a inside of a macrophage in the reticular zone (Fig. 2D).
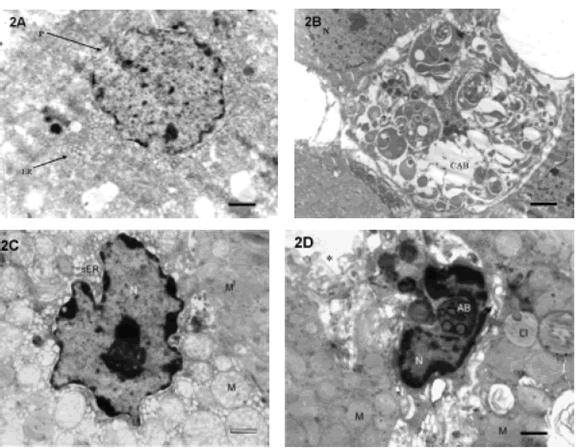
FIGURE 2. Electron micrographs of all zones of adrenal cortex from pregnant rats: A. Fascicular zone cell showing an apoptotic nucleus with chromatin fragmentation and loss of the nuclear membrane integrity (P). Typical cytoplasm of a steroids-synthesizing cell with abundant endoplasmic reticule (ER). Condensation and general disorganization signs are observed (bar =1 mm) (X 7,000). B. Glomerular zone cell with a cytoplasmatic apoptotic body (CAB). Different organelles and nuclear rests under the autophagocytosis process are observed. (N): normal nucleus of a nearby cell with intact membrane nuclear (bar = 2 mm). C: Reticular zone cell showing a shrunken nucleus (N) with condensed chromatin an altered relation of the membrane-attached chromatin and evident nucleolus. Alterated mitochondrias (M) and alterated smooth endoplasmic reticulum (sER). M1: unaffected mitochondria present in a neighbor cell with normal ultraestructure (bar = 1 mm) (X 8,000). D: Degraded apoptotic body (AB) inside of a macrophage (N: nucleus) in the reticular zone. Lipid droplet (LI) and mitochondrias (M) in adjacent nonaffected cells. (*): sinusoid capillary. (bar = 1 mm) (X 8,000).
When we analyzed the apoptotic index (A.I.) in the different zones of adrenal cortex, no significant differences were observed between adrenocortical zones from CR and ER groups at any of the three gestation days studied (Table 1). However, the A.I. in the three adrenocortical zones was highly influenced by the gestation day (p <0.0001) since it increased as gestation progressed in both CR and ER groups. Differences were significant in CR between 12 and 17, and 12 and 21 days (p<0.05); in ER, between 12 and 17, 17 and 21, and 12 and 21 days (p<0.05).
TABLE 1. Apoptotic index in adrenal cortex of rats during the second half of gestation

Effects of treatment and the gestation day on the total A.I. of adrenal cortex were significantly different in ER vs. CR at 12 th day (p<0.001) and 21st day of gestation (p<0.0001) (Fig. 3).

FIGURE 3. Total apoptotic index in the adrenal cortex of pregnant rats. Control rats (CR) vs. stressed rat (ER). **: highly significant differences (p< 0.001).
Discussion
The results showed these after applying chronic stress by intermittent and repeated IMO on the second half of gestation may be related to previous data reported by our research group. We showed an increase of more than 100% in the PRL plasmatic levels since day 19th to 21st in CR, while in ER the levels decreased 40% compared to day 12th. The decrease on day 21st in stressed rats was significant compared to controls on the same day and day 12th (Soñez et al., 1996).
Thus, the decrease in PRL levels observed at 21 days of gestation might stimulate apoptosis in the adrenal cortex since when its plasmatic levels are high, a lower A.I. was observed in ER compared to CR. Besides, the plasmatic levels of PRL together with lower corticosterone (CORT) plasmatic levels on day 21st (Romanini et al., 1999) might explain the lower A.I. found in CR rats compared to ER animals.
These hypotheses are supported by the fact that PRL was proved to promote proliferation, cell differentiation and cell death blocking induced by glucocorticoids (GC) (Krishnan et al., 2003). PRL inhibits the action of GC which produces the loss of mitochondria trans-membrane potential and consequently, the phosphatidylserine exposition and DNA fragmentation. PRL also promotes the expression of antiapoptotic bcl-2 protein. Besides, it has been postulated that PRL might inhibit a caspase that is activated by GC (Weimann et al., 1999).
Weimann et al. (1999) also demonstrated that the treatment of thymocytes with GC induces zeiosis, phosphatidylserine residues reorientation and transmembranous transport alteration as well as mitochondrial and plasmatic membranes potential loss. It was demonstrated that the GC-induced activation of caspase- 3 is a fundamental characteristic of the apoptotic process; the activation was inhibited in the presence of high PRL plasmatic levels (Krishnan et al., 2003).
These observations revealed that GC ability to induce membrane depolarization in target cells is directly related to its capacity to promote apoptosis, suggesting that under increased GC conditions, as it happens under stress, the increase in PRL plasmatic levels would be a physiological mechanism to achieve survival and adequate cellular function (Krishnan et al., 2003).
In previous studies performed by our research group, CORT plasmatic levels were determined 24 hours after stress, in the second half of gestation; significant differences were observed on day 21st when CORT values in the stressed mother rats were higher than those in control mother rats. Besides, the characteristic CORT hormonal profile in the second half of pregnancy was maintained in control mother rats as well as in stressed mother rats (Romanini et al., 1999).
Apoptotic index increase on day 21st of gestation in the ER group might be related to the increase in plasmatic CORT levels since they were significantly higher in ER compared to CR.
These results might be explained by the effect that GC exerts on insulin-like growth factor-1 (IGF-1). This factor is able to activate or repress specific determinant genes that promote cells survival, proliferation and cellular death when interacting with small hydrophobic molecules such as GC (Czech, 1989).
It was demonstrated that IGF levels are diminished as a response to nutrient restriction and/or hypoinsulinemia. Related to hypoinsulinemia and stress, it is already known that the metabolic activation caused by sympathetic medullary adrenal or pituitary-adrenal (PA) systems is opposite to insulin effect; thus, under stress conditions insulin secretion might be inhibited probably by a direct a-adrenergic effect at the level of pancreatic b-cells (Márquez et al., 2004).
On the other hand, GC produces an IGF masking, inhibition of the expression of survival genes such as bcl-2, and endonuclease activation which might lead to chromatin fragmentation and, as a consequence of all these facts, to an increase in the A.I. as well. Probably, protein p53 might be involved in apoptosis induction after DNA damage (Collins et al., 1994).
It is also suggested that plasmatic PRL levels diminished on day 21st of gestation in ER (Soñez et al., 1996) might cause apoptosis blocking liberation. Maternal and/or fetal GC liberated as a response to chronic stress would probable induce hormonal and metabolic changes in mothers. These changes would modify IGF synthesis or expression.
The activation of the HPA axis is common for all stressing situations and it is characterized by a rapid liberation of ACTH which stimulates GC synthesis in the fascicular zone of adrenal cortex. When the stimulus persists, even when it is highly intense, PA axis hormonal levels gradually return to normality. However, this pattern was displayed by ACTH but not by GC which remained high (Douglas et al., 2003).
The dissociation of ACTH-CORT suggests that some mechanisms besides than ACTH contribute to adrenocortical secretion.
In our experiment, the plasmatic ACTH levels were not determined but we were able to verify the CORT increase (Romanini et al., 1999). This dissociation might also explain the A.I. increase found in ER.
ACTH promotes adrenocortical cells survival. Respecting this fact when using in vivo and in vitro patterns to study the regulating cells survival factors, significant differences in the in vivo apoptosis pattern with hypophysectomized rats where the apoptosis was scarce were found. On the other hand, in vitro pattern and with absence of ACTH apoptosis produced rapidly (Carsia et al., 1996, 1997).
This difference may be due to the fact that apoptosis induced by hypophysectomy did not take place until ACTH concentration was decreased. On the other hand, ACTH injection in vivo attenuated apoptotic process via some anti-apoptotic mechanisms mediated by the hormone (Carsia et al., 1996).
All these findings allow us to hypotheize that cellular death in adrenal gland independently of ACTH might be finely regulated by the coordinated action of pituitary factors, intraglandulary factors liberated under stress situation such as catecholamines, vasoactive intestinal peptide and adrenal innervation. Thus, in hypophysectomized rats the intact innervation might regulate the localization and magnitude of apoptotic cellular death as a response to the ACTH absence (Carsia et al., 1996).
Controversial results have been reported when analyzing the apoptotic process in each zone of the adrenal cortex. Blanco et al. (2001), working under normal conditions, found apoptotic nuclei in the three adrenocortical zones but the highest number was found in the reticular zone. Carsia et al. (1998) found apoptotic nuclei only in the fascicular and reticular zones. Wolkersdorfer et al. (1996) detected apoptotic nuclei in the three adrenal cortex zones but the highest A.I. was found in the glomerular region.
In our work, apoptotic nuclei were found in the three zones studied in CR as well as in ER without significant differences. Differences were found in both CR and ER groups throughout gestation, independently of the zone.
According to our results, each adrenocortical zone behaves independently inregards to apoptosis and cellular proliferation. Besides, each one of them might be locally regulated without being affected by other zones (Wolkersdorfer et al., 1998).
In agreement with these results, apoptosis might represent a regulatory mechanism of tissue integrity which might allow the organism to face stressing situations as well as functional demands required for replication, differentiation and cellular migration. Thus, the occurrence of the apoptosis in the three adrenal cortex zones might guarantee the independent regulation of all stages of cellular development in the different cytophysiopathologic situations.
References
1. Blanco A, Monterde JG, Mendez A, Artacho-Perula E (2001). Stereological study of normal and apoptotic cell populations in the adrenal gland in calves. Cell Tissues Organs 169 (1):73- 80. [ Links ]
2. Carsia R, Macdonald G, Gibney J, Tilly K, Tilly J (1996). Apoptotic cell death in the rat adrenal gland: an in vivo and in vitro investigation. Cell and Tissue 283: 247-254. [ Links ]
3. Carsia R, Tilly K, Tilly J (1997). Hormonal modulation of apoptosis in the rat adrenal gland in vitro is dependent on structural integrity. Endocrine 7(3): 377-381. [ Links ]
4. Carsia R, Nagele R, Morita Y, Tilly K, Tilly J (1998). Models to elucidate of adrenal cell death. Endocr 24(3-4): 899-908. [ Links ]
5. Collins M, Perkins G, Rodriguez-Tarduchy G, Nieto MA, López- Rivas A (1994). Growth factors as survival factors: regulation of apoptosis. Bioessays 16: 133-138. [ Links ]
6. Czech MP (1989). Signal transmission by the insuline-like growth factors. Cell 59: 235-243. [ Links ]
7. Douglas JA, Brunton JP, Bosch JO, Russell AJ, Neumann DI. (2003). Neuroendocrine responses too stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinol 144(12): 5268-5276. [ Links ]
8. Ducsay CA, Hess DL, McClellan MC, Novy M (1991). Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in babbons. J Clin Endocrinol Metab 73: 385-395. [ Links ]
9. Krishnan N, Thellin O, Buckley DJ, Horseman ND, Buckley AR (2003). Prolactin suppress glucocorticoid-induced thymocyte apoptotic in vivo. Endocrinol 144(5): 2102-2110. [ Links ]
10. Márquez C, Nadal R, Armario A (2004). The hypothalamic pituitary adrenal and glucose reponses to daily repeated immobilisation stress in rats: Individual differences. Neuroscience 601-612. [ Links ]
11. Michajlovskij N, Lichardus B, Kvetñasnsky R, Ponrc J (1988). Effect of acute and repeated immobilization stress on food and water intake, urine output and vasopressin changes in rats. Endocrinol Exp 22: 143-157. [ Links ]
12. Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y (1994). A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinol 135: 431-438. [ Links ]
13. Romanini MC, Rodríguez N, Mugnaini MT, Rolando A, Soñez CA, Gauna H (1999). Effects of chronic stress by immobilization on the plasmatic levels of corticosterone in pregnant rats. Proc. Soc. Biol. Tucumán, Arg. (abst) Biocell 23(1). [ Links ]
14. Russell WMS, Burch RL (1959). The principles of Humane Experimental Technique 238pp London: Methuen. [ Links ]
15. Smyth D (1978). And Alternatives of to Animal Experiments. 218pp London: Scolar Press. [ Links ]
16. Soñez C, Mugnaini M, Bec. D, Rolando A, Romanini M, Díaz M, Rodríguez M, Gauna H (1996). Effects of chronic stress epon the plasmatic levels of PRL, FSH, LH, estradiol and progesterone in pregnant rats. Com Biol 14: 81. [ Links ]
17. Weimann E, Baixeras E, Zamzami N, Kelly P (1999). Prolactin blocks glucocorticoid induced cell death by inhibition the mitocondrial membrane. Leuk Res 23(8): 751-762. [ Links ]
18. Wolkersdorfer G, Bornstein-Ehrhart M, Brauer S, Marx C, Scherbaum W, Bornstein S (1996). Differential regulation of apoptosis in the normal human adrenal gland. Journald of Clinical Endocrinology and Metabolism 81: 4129-4136. [ Links ]
19. Wolkersdorfer G, Bornstein S (1998). Tissue remodelling in the adrenal gland. Biochemical Pharmacol 56: 163-171. [ Links ]
20. Yeasting R (1986). Selected morphological aspects of human suprarenal glands. In: The adrenal gland. Mulrow P.J. Ed., Elsevier. New York. pp. 45-63. [ Links ]
Received on September 6, 2005.
Accepted on July 6, 2006.














