Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.31 no.1 Mendoza Jan./Apr. 2007
Endogenous ADP-ribosylation of eukaryotic elongation factor 2 and its 32 kDa tryptic fragment
Kivanç Ergen,*, Muhammet Bektas¸**, Sina Gökçe** And Rüstem Nurten**
* Kocaeli University Medical Faculty, Department of Biophysics, 41380 Umuttepe, Izmit-Kocaeli, TURKEY.
** Istanbul University Istanbul Faculty of Medicine, Department of Biophysics, 34390 Çapa-Ístanbul, TURKEY
Address correspondence to: Dr. Kivanç Ergen. Kocaeli University Medical Faculty, Department of Biophysics, 41380 Umuttepe,Ízmit-Kocaeli, TURKEY. Phone: (+90 262) 303 84 97. E-mail: kergen@hotmail.com
ABSTRACT: Eukaryotic elongation factor 2 (eEF-2) can undergo ADP-ribosylation in the absence of diphtheria toxin. The binding of free ADP-ribose and endogenous transferase-dependent ADP-ribosylation were distinct reactions for eEF-2, as indicated by different findings. Incubation of eEF-2 tryptic fragment 32/33 kDa (32F) with NAD was ADP-ribosylated and gave rise to the covalent binding of ADP-ribose to eEF-2. 32F was revealed to be at the C-terminal by Edman degradation sequence analysis. In our study, the elution of 32F from SDS-PAGE was ADP-ribosylated both in the presence and absence of diphtheria toxin. These results suggest that endogenous ADP-ribosylation of 32F might be related to protein synthesis. This modification appears to be important for the cell function.
Key words: Eukaryotic elongation factor 2; Endogenous ADP-ribosylation; Protein synthesis; Trypsin digestion; Diphtheria toxin.
Introduction
Eukaryotic Elongation factor 2 (eEF-2) is one of the three protein factors involved in eukaryotic polypeptide chain elongation and promotes translocation in this process (Bermek, 1978). eEF-2 provides a unique site of attack for diphtheria toxin and Pseudomonas exotoxin in eukaryotic cell (Collier, 1967; Honjo et al., 1971; Iglewski and Kabat, 1975). These toxins inactivate eEF-2 by catalyzing ADP-ribosylation of its diphthamide residue (Van Ness et al., 1980) thereby inhibiting protein synthesis (Pappenheimer, 1977).
It has been previously argued that the toxin effect may simulate a normal cellular mechanism (Collier, 1975). Endogenous ADP-ribosyltransferase activity specific for eEF-2 has been reported in mammalian systems (Lee and Iglewski, 1984; Sitikov et al., 1984; Sayhan et al., 1986), and it appears to be an inherent property of eEF-2 (Sayhan et al., 1986). This activity, which co-migrates with eEF-2 purification, has been implicated as an inherent property of the factor (Sayhan et al., 1986).
Another line of research revealed that eEF-2 is also specifically phosphorylated by Ca/calmodulin-dependent kinase (protein kinase III or eEF-2 kinase) (Palfrey, 1983; Nairn et al., 1985; Ryazanov et al., 1988; Zamboni et al., 1991). The phosphorylated form of eEF2 is also inactive in protein synthesis. These data suggest that eEF-2 is an important site for cellular control mechanism through posttranslational modifications, as underlined by the presence of modified variants of eEF2 in cell lysates (Celis et al., 1990; Marzouki et al., 1989). Increases in the relative proportions of phosphorylated variant of eEF-2 during mitosis (Celis et al., 1990) and of the ADP-ribosylated variant in aged human cell cultures (Riis et al., 1990) have been reported. Another eEF-2-mediated control mechanism for elongation appears to be the cellular proteolytic degradation of eEF-2, as indicated by the presence of varying amounts of ADP-ribosylatable fragments in different cell types and different stages of cell growth (Zamboni et al., 1991; Giovane et al., 1987). It has been suggested that the binding of free ADP-ribose, diphtheria toxin and endogenous transferase-catalysed ADP-ribosylations in the presence of NAD are distinct reactions (Bektas¸ et al., 2006). Bektas¸ et al. (2006) showed that both types of endogenous ADP-ribosylation give rise to inhibition of polyphenylalanine synthesis.
In this report, we prove that incubation of eEF-2 tryptic fragment 32/33 kDa (32F) with NAD was ADPribosylated. Endogenous ADP-ribosylation of 32F was revealed to be at the C-terminal by Edman degradation. This site appears to be important for the functional integrity of the factor.
Material and Methods
eEF-2 purification
All reagent grade chemicals were obtained from the Sigma Chemical Co. (St. Louis, USA). The diphtheria toxin (DT) was a gift from the Refik Saydam Institute in Ankara. eEF-2 was purified from the rat livers as previously described (Nurten and Bermek, 1980). Briefly, finely minced rat livers were homogenized in 50 mM Tris-HCI, at pH 7.4, 25 mM KCI, 5 mM MgCI2, 7 mM mercaptoethanol and 250 mM sucrose, which is also used as dialysis buffer (DB), with a Potter homogenizer. The homogenate was subjected to differential centrifugation; following the initial centrifugation for 20 min at 30,000 xg, the postmitochondrial supernatant was centrifuged for 2h at 130,000 xg. eEF-2 was obtained from the rat liver postribosomal supernatant by adsorption to hydroxyapatite and subsequent elution. eEF-2 was eluted between 50 mM and 150 mM potassium phosphate pH 7.0. eEF-2 was further purified by chromatography on DEAE-cellulose (DE52, Whatman) and eluted between 15-65 mM KCl in 50 mM Tris-HCl, pH 7.4, 7 mM mercaptoethanol and 0,1 mM EDTA. The eEF-2 proteins were then fractionated on phosphocellulose (P11, Whatman) and eluted between 50 mM and 250 mM KCl/phosphate buffer, pH 6.8 and 7 mM 2-mercaptoethanol.
ADP-ribosylation
Molar amounts of EF-2 were determined by ADPribosylation for 10 min at 20ºC in the presence of 50 mM Tris HCl, pH 7.4, 7 mM mercaptoethanol and 5 μM [14C] NAD labelled in adenine moiety (specific activity 252 mCi/mmol, Amersham Pharmacia Biotech UK), and 120 μg/ml diphtheria toxin (Nurten and Bermek, 1980). Endogenous ADP-ribosylation was carried out for 1 h at 20ºC in the presence of the same components except the diphtheria toxin. Aliquots from the reaction mixtures were applied to GF/A (Whatman) filters, which were washed successively in cold 5% TCA, ether-ethanol and ether. After being dried, the filters were transfer red to vials containing 5 ml 0.4% 2.5-diphenyloxazol in toluene, and TCA-precipitated radioactivity was determined in a liquid scintillation spectrometer (Packard Tri-Carb 1000TR) (Nurten and Bermek, 1980).
Trypsin digestion and identification of radiolabeled fragment
For partial digestion, eEF-2 was incubated in 20 mM Tris-HCl, pH 7.4, 100 mM KCl, 0.1 mM EDTA, 5 mM mercaptoethanol and 10% glycerol with trypsin (at a molar trypsin/EF-2 ratio of 1/500) for 30 min at 37ºC. The reaction was stopped by adding a stoichiometric amount of soya bean inhibitor and subsequent denaturation of the sample as described (Bilgin et al., 1990). Alternatively, toxin-mediated and endogenously ADPribosylated eEF-2 was incubated under the same condition for 30 min. Tryptic peptides from endogenously ADP-ribosylated eEF-2 separated by SDS-PAGE (sodium dodecylsulfate polyacrylamide gel electrophoresis) were electrophoretically transferred to PVDF membrane and, thereafter, subjected to sequence analysis by Edman degradation (car ried out by Eurosequence Netherland using an automatic sequenator Model 494 Procise or Model 477A, Applied Biosystems).
Eluation of eEF-2 and 32F and zymographic in situ ADP-ribosylation
eEF-2 and 32F eluted from SDS-PAGE were renatured as described (Sayhan et al., 1986). Briefly, the slices were homogenized in 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 0.1 mM EDTA and 0.1 mg/ml BSA. The homogenates were left at room temperature overnight, followed by centrifugation through siliconized glasswool for 15 min at 2000 xg. The centrifugates were precipitated with 4 volumes of cold (-20ºC) acetone, dissolved in 100 μl 6 M guanidine hydrochloride and renaturated by dialysis against 50 mM Tris HCl, pH 7.4, 150 mM KCl, 1 mM DTE, 0.1 mM EDTA, 0.1mg/ml BSA and 2% glycerol. From each sample 10 μl aliquots were assayed for ADP-ribosylation in the presence or absence of diphtheria toxin. Zymographic in situ ADPribosylation following SDS-PAGE was performed according to (Hager and Burgess, 1980; Nilsson and Nygard, 1985). Then eEF-2 was renaturated in 20 volumes of 2.5% Triton X-100 for two cycles of 30 min and in H2O for an additional 30 min, and the gel was incubated for 1 h at room temperature in 1.5 ml of zymographic reaction mixture containing 100 mM phosphate, pH 7.5, 1 mM DTT, 400 mM NaCl, 2 mg/ml histone, 50 μM ADP-ribose and 2 μM [14C] NAD labelled in the adenine moiety. The reaction was stopped in 50% TCA, 0.1% Coomassie Brilliant Blue.
Electrophoretic and autoradiographic analysis
SDS-PAGE analysis was performed as described (Laemmli, 1970). β-galactosidase, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase and soya bean inhibitor were used as molecular weight standards. The proteins were stained with Coomassie Brilliant Blue, destained in 7% acetic acid and 30% methanol and exposed to Kodak X-Omat K films.
Results
Electrophoretic and autoradiographic analysis of ADPribosylated eEF-2 and its tryptic fragments
Mild trypsin digestion of eEF-2 (and of ADPR-EF-2) resulted in SDS-PAGE with the appearance of major polypeptides of about 66, 47, 32/33 and 23 kDA, plus a number of minor products (Fig. 1). Autoradiographic analysis and radioactive counting revealed that, in addition to ADPR-EF-2 corresponding to the 97 kDa, the 32/33 kDa polypeptide (32F) carried the radioactive label (Fig. 1 and Fig. 2). Both native eEF-2 and ADPribosylated eEF-2 (whether mediated by diphtheria toxin or by endogenous ADP-ribosylation) gave the same profile with trypsin digestion (Fig. 2, lane 1 and 3). The eluates corresponding to intact eEF-2 and to 32F were also ADP-ribosylated both in the presence and absence of diphtheria toxin, and both ADP-ribosylations were resistant to cold TCA-treatment and the radioactive label was retained after SDS-PAGE, which shows the covalent binding character. (Fig. 1 and Fig. 2A, lane 3).
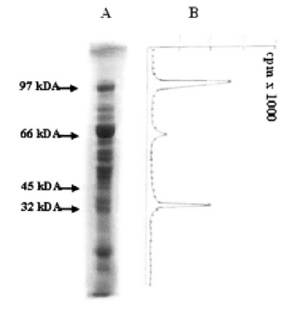
FIGURE 1. Mild trypsin digestion of diphtheria toxin ADP-ribosylated eEF-2. 60 μg of ADP-ribosylated eEF- 2 was partially digested at 37ºC for 30 min. After separation by SDS-PAGE, 1 mm slices were incubated at 50ºC for 24h in 3% H2O2. The radioactivity of 1 mm slices was counted. (A) ADP-ribosylated eEF-2 after trypsin treatment. (B) Radioactivity of slices.
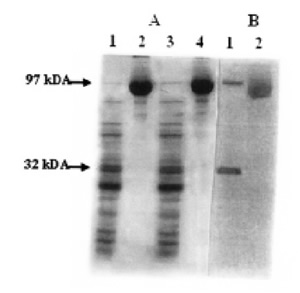
FIGURE 2. Mild trypsin digestion of endogenous ADPribosylated eEF-2. 60 μg eEF-2 and 60 μg ADPribosylated eEF-2 were partially digested at 37ºC for 30 min. After separation by SDS-PAGE, autoradiography was applied. (A) Lane 1- eEF-2 (native) + trypsin; Lane 2- eEF-2 (native); Lane 3- ADP-ribosylated eEF-2 (by endogenous ADP-ribosylation) + trypsin; Lane 4- ADPribosylated eEF-2. (B) Lanes 1 and 2 show the autoradiography corresponding to lanes 3 and 4 of panel A, respectively.
Zymographic in situ ADP-ribosylation
Endogenous ADP-ribosylation of the 32F was also shown by zymographic in situ reaction, which gave rise to the radioactive labeling of the same band(s) (Fig. 3).
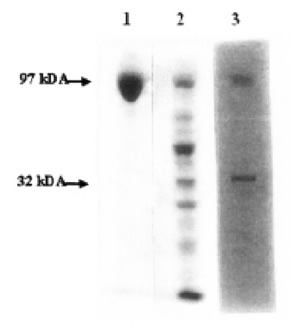
FIGURE 3. Zymographic and autoradiographic analysis of mild trypsin digestion of eEF-2. 60 μg eEF-2 was partially digested for 30 min at 20ºC. After separating by SDSPAGE, Zymography (in-situ hybridization) was applied. Lane 1- Control eEF-2; Lane 2- Zymography of eEF-2 after trypsin treatment; Lane 3- Autoradiography of 2.
Edman degradation sequence analysis
N-terminal sequence analysis of 32/33F revealed the presence of two fragments with the N-termini corresponding to L526 and S529 on eEF-2, respectively. Therefore, in this study, we proved that endogenous ADP-ribosylation of eEF-2 with NAD takes place at the C-terminal part of eEF-2, namely at 32F.
Discussion
eEF-2 is known to undergo ADP-ribosylation in the absence of diphtheria toxin (Lee and Iglewski, 1984; Sitikov et al., 1984; Sayhan et al., 1986). An endogenous ADP-ribosyltransferase has been implicated to account for this reaction (Lee and Iglewski, 1984; Bermek, 1976; Iglewski and Dewhurst, 1991). However, ADP-ribosylation has also been observed with purified eEF-2 fractions and under conditions excluding the presence of such presumptive cellular activity (Iglewski, 1994). ADP-ribosylation of eEF-2 appears to be a common cellular event. Indeed, eEF-2 fractions reveal varying degrees of endogenous ADP-ribosylation, which appears to increase with aging (Marzouki et al., 1989). Recently, it was shown that ADP-ribosylation of eEF-2 takes place under physiological conditions. However, H2O2-promoted oxidative stress gave rise to an increase that is two times greater than the in vivo labeling eEF-2 in a K562 cell culture and was accompanied by a loss of eEF-2 activity in polypeptide chain elongation (Bektas¸ et al., 2005). It has been suggested that the binding of free ADP-ribose, diphtheria toxin and endogenous trans-ferase-catalysed ADP-ribosylations in the presence of NAD are distinct reactions (Bektas¸ et al., 2006). Bektas¸ et al. (2006) showed that both types of endogenous ADPribosylation give rise to inhibition of polyphenylalanine synthesis.
Mild trypsin digestion of eEF-2 (and of ADPribosylated eEF-2) resulted in SDS-PAGE patterns similar to those previously reported (Nilsson and Nygard, 1985) (Fig. 1). 32/33 kDa polypeptide (32F) carried the radioactive label, a finding seemingly consistent with a previous report (Fig. 2B) (Nilsson and Nygard, 1985). These findings extended the scope of the former data showing that the fragments arising from the proteolytic cleavage of eEF-2 can be ADP-ribosylated in the presence of diphtheria toxin (Giovane et al., 1987). As indicated by additional data (not shown), purified fractions of eEF-2 underwent a specific breakdown with the appearance of minor polypeptides with a molecular range of 20-36 kDa. A report on the presence of an eEF-2-specific proteolytic activity (Servillo et al., 1988) is consistent with these findings.
In our study, ADP-ribosylation of 32F appeared to result in the formation of covalent linkage on the basis of its product to cold TCA-treatment as well as retention of bound radioactive label after SDS-PAGE, as is the case with intact eEF-2, as has been previously reported (Bektas¸ et al., 2005). In that report, binding of free ADP-ribose, and binding of ADP-ribose which took place in the presence of NAD due to endogenous ADPribosyltransferase action, also appeared to result in the formation of covalent linkage.
In the present study, it was also shown that the C-terminal part of eEF-2, which is 32kDa, carried the ADP-ribosylated fragment. This finding is consistent with a previous report that the sites involved in this modification by the toxin and endogenous transferase have been found to reside on the same tryptic peptide from the carboxyl terminal region of the factor (Fendrick et al., 1992). This may suggest an alternative function of 32F apart from the intact eEF-2. GTP interaction was shown to be at 158-161 amino acid residues of eEF-2 (McKeehan, 1972); therefore, it can be excluded the likely interaction of NAD at the GTP binding site, since 32F has N-termini corresponding to L526 and S529. Moreover, as previously shown, by cleaving with the tryptophan-specific reagent N-chlorosuccinimide, the site of ADP-ribosylation by diphtheria toxin, could be located at a distance of 40-60 kDa from the GTP-bind-ing site and about 4-11 kDa from the nearest terminus (Nilsson and Nygard, 1985) and diphtheria toxin-cata-lyzed ADP-ribosylation takes place at histidine residue of codon 715 that is modified post-translationally to diphthamide (Omura et al., 1989; Kohno and Uchida, 1987). It was shown that purified eEF2 recovered from SDS-PAGE can be radiolabeled by using [14C]NAD (Sayhan et al., 1986). In agreement with that report, it was suggested that eEF2 can contain an ADPribosyltransferase site, which can be activated in vitro and give rise to auto-ADP-ribosylation (Iglewski and Dewhurst, 1991). The finding of this study that the elution of 32F from SDS-PAGE was ADP-ribosylated both in the presence and absence of diphtheria toxin extended this view.
ADP-ribose generated from NAD and/or poly ADP-ribose under glycohydrolase action can bind to, following an Amadori rearrangement, a protein amide group under formation of a Schiff base (Fendrick and Iglewski, 1989; Parrado et al., 1999; Vary et al., 1994; Ayala et al., 1996; Jacobsen et al., 1994). This ketoamine adduct can, in turn, give rise under oxidation to protein glyocoxidation products, i.e. advanced glycosylation end products (AGE) (Khalifah et al., 1996; Cervantes-Laurean et al., 1993). Protein glycation by pentoses and hexoses appears to play a role in the physiopathology of a number of diseases such as diabetes as well as in oxidative stress and aging (Jacobson et al., 1997). Our work does not explain whether 32F was ADP-ribosylated by binding free ADP-ribose or binding ADP-ribose under the endogenous transferase action in the presence of NAD.
The modification of eEF-2 within the context of such a condition, the formation of ADP-ribosylated 32F, may well be related to protein synthesis as in the case of ADP-ribosylated eEF-2. Increased NAD/ADP-ribose turnover following DNA damage might depress protein synthesis in the cell by ADP-ribosylation. This also shows the unique binding reaction of ADP-ribose to eEF-2.
In conclusion, the results presented in this report attest to the presence of ADP-ribosylation site on 32F at the C-terminal half of eEF-2. Work is in progress on the elucidation of the molecular mechanism of endogenous ADP-ribosylation and characterization of the sites involved.
Acknowledgement
This work was supported by the Research Fund of the Istanbul University (Grant 1731-15082001, 181/ 15012004 and T-366/190397).
References
1. Ayala A, Parrado J, Bougria JM, Machado A (1996). Effects of oxidative stress, produced by cumene hydroperoxide, on the various steps of protein synthesis. J Biol Chem. 271(38): 23105-23110. [ Links ]
2. Bektas¸ M, Akçakaya H, Aroymak A, Nurten R, Bermek E (2005). Effect of oxidative stress on in vivo ADP-ribosylation of eukaryotic elongation factor 2. Int J Biochem Cell Biol. 37: 91-99. [ Links ]
3. Bektas¸ M, Nurten R, Ergen K, Bermek E (2006). Endogenous ADP-ribosylation for eukaryotic elongation factor 2: evidence of two different sites and reactions. Cell Biochem Funct. 24(4): 369-380. [ Links ]
4. Bermek E (1976). Interactions of adenosine diphosphateribosylated elongation factor 2 with ribosomes. J Biol Chem. 251(21): 6544-6549. [ Links ]
5. Bermek E (1978). Mechanisms in polypeptide chain elongation on ribosomes. Prog Nucleic Acid Res Mol Biol. 21: 63-100. [ Links ]
6. Bilgin N, Sayhan O, Bermek E (1990). Binding of periodate-oxi-dized guanine nucleotides to eukaryotic elongation factor 2. Biochim Biophys Acta. 1048: 217-222. [ Links ]
7. Celis JE, Medsen P, Ryazanov AG (1990). Increased phosphorylation of elongation factor 2 during mitosis in transformed human amnion cells correlates with a decreased rate of protein synthesis. Proc Natl Acad Sci U S A. 87: 4231-4235. [ Links ]
8. Cervantes-Laurean D, Minter DE, Jacobson EL, Jacobson MK (1993). Protein glycation by ADP-ribose: studies of model conjugates. Biochemistry. 32: 1528-1534. [ Links ]
9. Collier RJ (1967). Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Biol Chem. 25: 83-98. [ Links ]
10. Collier RJ (1975). Diphtheria toxin: mode of action and structure and its hydrolysis products. Bacteriol Rev. 39: 54-85. [ Links ]
11. Fendrick JL, Iglewski WJ (1989). Endogenous ADP-ribosylation of elongation factor-2 in polyoma virus-transformed baby hamster kidney cells. Proc Natl Acad Sci U S A. 86: 554-557. [ Links ]
12. Fendrick JL, Iglewski WJ, Moehring JM, Moehring TJ (1992). Characterization of the endogenous ADP-ribosylation of wild-type and mutant elongation factor 2 in eukaryotic cells. Eur J Biochem. 205(1): 25-31. [ Links ]
13. Giovane A, Servillo L, Quagliuolo L, Balestrieri C (1987). Purification of elongation factor-2 from human placenta and evidence of its fragmentation patterns in various eukaryotic sources. Biochem J. 244: 337-344. [ Links ]
14. Hager DA, Burgess RR (1980). Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 109(1): 76-86. [ Links ]
15. Honjo T, Nishizuka Y, Kato I, Hayaishi O (1971). Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J Biol Chem. 246(13): 4251-4260. [ Links ]
16. Iglewski BH, Kabat D (1975). NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci U S A. 72(6): 2284-2288. [ Links ]
17. Iglewski WJ (1994). Cellular ADP-ribosylation of elongation factor-2. Mol Cell Biochem. 138(1-2): 131-133. [ Links ]
18. Iglewski WJ, Dewhurst S (1991). Cellular mono(ADP-ribosyl) transferase inhibits protein Synthesis. FEBS Lett. 283(2): 235-238. [ Links ]
19. Jacobson EL, Cervantes-Laurean D, Jacobson MK (1994). Glycation of proteins by ADP- Ribose. Mol Cell Biochem. 138(1-2): 207-212. [ Links ]
20. Jacobson EL, Cervantes-Laurean D, Jacobson MK (1997). ADP-ribose in glycation and glycoxidation reactions. Adv Exp Med Biol. 419: 371-379. [ Links ]
21. Khalifah RG, Todd P, Booth AA, Yong SX, Mott JD, Hudson BG (1996). Kinetics of nonenzymatic glycation of ribonuclease A leading to advanced glycation end products. Paradoxical inhibition by ribose leads to facile isolation of protein intermediate for rapid post- Amadori studies. Biochemistry. 35(15): 4645-4654. [ Links ]
22. Kohno K, Uchida T (1987). Highly frequent single amino acid substitution in mammalian elongation factor 2 (EF-2) results in expression of resistance to EF-2-ADP-ribosylating toxins. J Biol Chem. 262(25): 12298-305. [ Links ]
23. Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(5259): 680-685. [ Links ]
24. Lee H, Iglewski WJ (1984). Cellular ADP-ribosyltransferase with the same mechanism action as diphtheria toxin and Pseudomonas toxin. Proc Natl Acad Sci U S A. 81(9): 2703-2707. [ Links ]
25. Marzouki A, Lavergne JP, Reboud JP, Reboud AM (1989). Heterogeneity of native rat liver elongation factor 2. FEBS Lett. 255(1): 72-76. [ Links ]
26. McKeehan W (1972). The ribosomal subunit requirements for GTP hydrolysis by reticulocyte polypeptide elongation factors EF1 and EF-2. Biochem Biophys Res. 48(5): 1117-1122. [ Links ]
27. Nairn AC, Bhagat B, Palfrey HC (1985). Identification of calmodulin-dependent protein kinase-III and its major Mr 100.000 substrate in mammalian tissues. Proc Natl Acad Sci U S A. 82(23): 7939-7943. [ Links ]
28. Nilsson L, Nygard O (1985). Localization of the sites of ADPribosylation and GTP binding in the eukaryotic elongation factor EF-2. Eur J Biochem. 148(2): 299-304. [ Links ]
29. Nurten R, Bermek E (1980). Interactions of elongation factor 2 (EF-2) with guanine nucleotides and ribosomes. Eur J Biochem. 103(3): 551-555. [ Links ]
30. Omura F, Kohno K, Uchida T (1989). The histidine residue of codon 715 is essential for function of elongation factor 2. Eur J Biochem. 180(1): 1-8. [ Links ]
31. Palfrey HC (1983). Presence in many mammalian tissues of an identical major cytosolic substrate (Mr 100.000) for calmodulin dependent protein kinase. FEBS Lett. 157(1): 183-190. [ Links ]
32. Pappenheimer AM Jr. (1977). Diphtheria toxin. Annu Rev Biochem. 46: 69-94. Review. [ Links ]
33. Parrado J, Bougria MA, Ayala Castano A, Machado A (1999). Effects of aging on the various steps of protein synthesis fragmentations of elongation factor-2. Free Radic Biol Med. 26(3-4): 362-370. [ Links ]
34. Riis B, Ratton SIR, Derventzi A, Clark BFC (1990). Reduced levels of ADP-ribosylatable elongation factor-2 in aged and SV40-transformed human cell cultures. FEBS Lett. 266(1-2): 45-47. [ Links ]
35. Ryazanov AG, Shestakova EA, Natapov PG (1988). Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 334(6178): 170-173. [ Links ]
36. Sayhan O, Özdemirli M, Nurten R, Bermek E (1986). On the nature of cellular ADP- ribosyltransferase from rat liver specific for elongation factor II. Biochem Biophys Res Commun. 139(3): 1210-1214. [ Links ]
37. Servillo L, Quagliuolo L, Balestrieri C, Giovane A (1988). Evidence of a yeast proteinase specific for elongation factor 2. FEBS Lett. 241(1-2): 257-260. [ Links ]
38. Sitikov AS, Davydova EK, Ovchinnikov LP (1984). Eukaryotic elongation factor-2 loses its non-specific affinity for RNA and leaves polyribosomes as a result of ADP- ribosylation. FEBS Lett. 176(2): 406-410. [ Links ]
39. Van Ness, BG, Howard JB, Bodley JW (1980). ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 255(22): 10710-10716. [ Links ]
40. Vary TC, Nairn A, Lynch CJ (1994). Role of elongation factor 2 in regulating peptide chain elongation in the heart. Am J Physiol. 266(4 Pt 1): 628-634. [ Links ]
41. Zamboni M, Brigotti M, Montanora L, Sperti S (1991). Elongation factor 2 from Artemia salina embryos and its affinity for ribosomes. Eur J Biochem. 200(1): 13-18. [ Links ]
Received on May 29, 2006.
Accepted on December 6, 2006.














