Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.31 no.1 Mendoza Jan./Apr. 2007
Effects of prenatal stress on male offspring sexual maturity
Nancy Rodríguez, Nora Mayer, And Héctor F. Gauna
Orientación Fisiología Animal. Departamento de Biología Molecular. Facultad de Ciencias Exactas Físico-Químicas y Naturales. Universidad Nacional de Río Cuarto. Córdoba. Argentina.
Address correspondence to: Dra. Nancy Rodríguez. Orientación Fisiología Animal, Departamento de Biología Molecular, Facultad de Ciencias Exactas, Físico-Químicas y Naturales. Universidad Nacional de Río Cuarto. Ruta 8, Km 601. (5800) Río Cuarto, Córdoba, ARGENTINA. E-mail: nmarioli@exa.unrc.edu.ar
ABSTRACT: Prenatal stimulations have been shown to have long-term effects on at reproductive activity. We evaluated the influence of the prenatal stress on the hypothalamic-pituitary-gonad (HPG) axis in male offsprings from mothers with high number of offsprings per litter (HNL) and low number of offsprings per litter (LNL) after hypothesizing that the number of offsprings per litter may modify the effect of the prenatal stress on the HPG of adult offsprings. Pregnant Wistar rats were used for this study. Immobilization (IMO) stress was used, 30 min, 3 times per week, from the 5th to 21st day of pregnancy. The weight of adrenal and gonads, and the corticosterone (COR), testosterone (TES) and luteinizing hormone (LH) plasmatic levels were analyzed in the male offspring at 30, 45 and 70 days of age. The offspring males coming from LNL showed a decrease in testicle weight and TES levels, without changes in the plasmatic LH levels. However, the offspring of HNL showed a decrease of LH levels. It is possible to conclude that in LNL prenatal stress would produce alterations to gonadal level, while in HNL the effect of stress would be evident at pituitary level.
Key words: Rats; Prenatal stress; Reproduction; Corticosterone; Testosterone.
Introduction
The survival of organisms depends on the maintenance of a constant internal body environment, or homeostasis. In several physiological conditions, the hypothalamic-pituitary-adrenal axis actively responds to both physical and psychological stress stimuli (Chousos et al., 1988). In pregnant rats, the hypothalamic-pituitary-adrenal axis response is reduced during the last third of pregnancy. This response is manifested as a decrease in the levels of corticotropin-releasing-hormone (CRH), adrenocorticotropic (ACTH) and corticosterone (COR) hormones in animals exposed to prenatal stress (Fride and Weinstock, 1984). This attenuated response of the HPA axis during pregnancy may provide a protective mechanism during the development of the fetus axis (Neumann et al., 1998). Plasmatic levels of progesterone and gonadotropins were shown to decrease in pregnant females exposed to restrain stress (Kinsley and Svare, 1986). Inhibition of progesterone secretion in rat during gestation could be due to the luteolitic effect of ACTH and COR, and it may be the cause of fetal loss during the first period of pregnancy. Maternal hormone imbalance affects the fetus, producing sexual changes in adult offsprings (Weisz and Ward, 1980; Kinsley and Svare, 1986). This effect can be blocked in pregnant rats with more than ten conceptus (Kato et al., 1979). In fact, evidences indicate that rats with more than ten conceptus and high levels of progesterone are more resistant to stressful environmental conditions than those with only one conceptus (Sugino et al., 1991).
As there is no direct neural connection between the fetus and its mother, the maternal hormones should mediate the changes in the neuroendocrine system of the fetus induced by maternal stress. These hormones are COR, catecholamines, ACTH, and β-endorphins, which reach the fetal brain through the placenta (Fisher and Brown, 1991).
Chronic immobilization (IMO) stress induces a quick fall of testosterone (TES) hormone and reduces the response of the Leydig cells to LH. This inhibiting effect induced by stress could be mediated by several substances, such as endorphins, glucocorticoid and catecholamines (Charpenet et al., 1982). Other authors (Orr and Mann, 1990) observed that the greatest inhibiting capacity of chronic stress on the secretion of TES, without constant changes in the luteinizing (LH) hormone, would be due to an inhibition of the testicular steroidogenic, probably mediated by catecholamines (Collu et al., 1984) or glucocorticoid (Closet et al., 1986).
Sexual differentiation of the brain depends on the presence or absence of androgens during the early stages of the perinatal development. The levels of TES on days 18-19 of gestation (Ward and Weisz, 1980) are critical for sexual behavior and differentiation, gonadal secretion and several morphologic indexes. There is evidence that the sexual differentiation of the brain is also intimately related to the estrogen levels derived from the aromatization of the circulating TES (Chantal et al., 1996).
It has been hypothesized that prenatal stress disrupts the normal maternal-fetal hormonal environmental and suppresses the fetal TES peak on gestational days 18 and 19, a peak necessary for later expression and maintenance of male sexual behavior (Menéndez-Patterson et al., 1982; Ward and Weisz, 1984).
Considering that the number of offsprings per litter could modify the effects induced by stress on the hypothalamic-pituitary-gonad axis, the objective of the present work was to determine the influence of prenatal stress on the male's sexual maturity evaluating the gonadal-somatic index (IGS) and the activity of the hypo-thalamic-pituitary-gonadal axis through TES and LH levels in male offsprings from mothers with low number of offsprings per litter (LNL) and high number of offsprings per litter (HNL).
Materials and Methods
Albino Wistar rats of about 300 g body weight and four month of age were housed with ad libitum access to food and water, in constant light/dark cycle (lights on at 7 hr, and off at 19 hr), temperature (22 ± 2ºC), and humidity. Male and virgin female pairs were housed together overnight. The next morning, a vaginal smear was obtained and examined microscopically for the presence of sperm (day 1 of pregnancy). This process was repeated until sperm was found in the animal's vagina.
Pregnant rats were separated into two groups: a control group without stress, and an experimental group subjected to chronic IMO stress (Michajlovskij et al., 1988) for 30 minutes at 10 a.m. from day 7 to day 21 of pregnancy, three times a week.
The litters, experimental: (E) and control: (C), were separated into groups according to the number of offsprings per litter: HNL (E = 12.2 ± 0.46; C = 11.3 ±0.37) and LNL (E = 6.30 ± 0.75; C = 7.1 ± 0.70). The offsprings were weaned 21 days after birth, and two offsprings (male and female) were housed together for the rest of the study. This method contributes to the sexual development of the male. Both animals were separated before sexual maturity.
Animals
The number of offsprings was recorded at birth. All the determinations were performed in male offsprings at 30, 45 and 70 days of age, considering the litter size form which each offspring came from. COR, TES and LH levels were determined in heparin plasma samples. Control and stressed animals were sacrificed by decapitation. Plasma was stored at -20ºC.
Corticosterone assay
Corticosterone concentrations were measured by radioimmunoassay (Krey et al., 1975) wing a sheep antibody with high specificity (TECNOLAB). The Corticosterone standards were supplied by Sigma Chemical CO. The radioactive steroids were (0.25mCi) corticosterone, [1,2,6,7-3H (N)] - 3.0 TBq/mmol (80.00 Ci/mmol). 0.25 ml of ethanol. Sciences, Inc. Boston, MA. The intra and interassay coefficients of variation were 8.8%.
Testosterone assay
Determinations of testosterone levels were carried out wing the Coated Tube 125 I RIA Kit, COAT-A-COUNT Total Testosterone. DPC-Diagnostic Products Corporation, Los Ángeles CA.
Luteinizing hormone assay
Plasma LH concentration were measured using the RIA Kit for rat LH elaborated by Dr. A. F. Parlow, Director of Pituitary Hormone & Antisera Center, Harbor-UCLA Medical Center. National Institute of Diabetes & Kidney Diseases (NIDDK). The kit provided the following reagents:
1) RAT LUTEINIZING HORMONE ANTIGEN, highly purified, for iodination, NIDDK-rLH-I-9 (AFP-10250C)100 μg-ampoule, lyophilized.
2) RAT LUTEINIZING HORMONE ANTISERUM (rabbit), NIDDK-anti-rLH-S-11 1.0 ml, 1:70 dilm., lyophilized, in 2% normal rabbit serum (NRS) in phosphosaline buffer (PBS).
3) RAT LUTEINIZING HORMONE REFERENCE PREPARATION, NIDDK-rLH-RP-3 (AFP-7187B) for use as "cold standard" only. 10 mg-ampoule, lyophilized in 1 ml of 1% Bovine serum Albumin (BSA) in PBS.
The sample was counted in automatic gamma, WIZARD WALLAC, Mod 1470. The sensibility of the LH assay was 0.3 pg/tube, with 4.2% intra assay variability.
The adrenal-somatic and the gonadal-somatic indexes were calculates as follows:

Statistical analysis
The results were analyzed by three-way ANOVA; prenatal treatment (Stress vs Control), days of pregnancy and number of offsprings (LNL vs HNL). A p < 0.05 were considered. Was accepted as statistically significant. Student-Newman-Keuls post hoc test was performed as required.
Results
Our studies showed that, in rat, body weight at birth and 70 days of age were not affected by prenatal stress (Table 1).
ANOVA demonstrated a significant effect in the following parameters: offspring number (F (1,84) = 4.55, p = 0.035); treatment (F (1,84) = 38.08, p = 0.001) and time (F (2,84) = 12.75, p = 0.001). A significant interaction between offspring number vs. treatment was observed (F (1,84) = 6.04, p = 0.015). In the LNL group the adrenal-somatic index was increased in prenatal stressed offspring compared with their controls, while differences were not significant in the HNL group (Fig. 1).

FIGURE 1. Adrenal-somatic index of prenatal stressed and controls offspring rats from LNL and HNL. The results are expressed as mean ± SEM, with an n = 8 / bar. * p < 0.05: Stress vs. Control.
TABLE 1.
Body weight at birth and 70 days of age

The statistical analysis also revealed significant effects of the treatment (F (1,60) =7.003 p = 0.0103) and time (F(1,60) =4,90 p = 0.0106) on the plasma corticosterone, as well as significant interaction between these factors.
Differences of COR levels between stressed and control animals were significant in 70-day offsprings in LNL group. The same tendency was observed in HNL; the differences however, were not significant (Fig. 2).
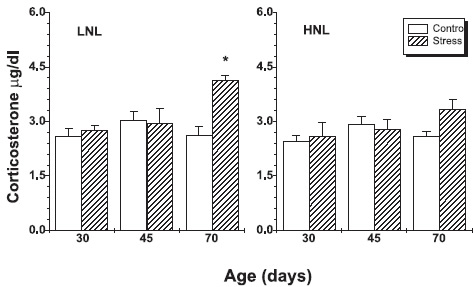
FIGURE 2. Plasma corticosterone levels of prenatal stressed and controls offspring rats from LNL and HNL. The results are expressed as mean ± SEM, with an n = 6 / bar. * p < 0.05: Stress vs. Control.
ANOVA showed, in addition, significant effects of offspring number (F (1,84) = 4.45, p = 0.037), treatment (F (1,854) = 24.69, 0.001) and time (F (2,84) = 79.12, p = 0.001) on gonadal-somatic index, as well as interaction of the three factors (F (2,84) = 10.74, p = 0.001).
In control animals, a significant increase in the gonadal-somatic index was observed in offsprings from LNL group in comparison with HNL offsprings, at 45 and 70 days of age (Fig. 3).

FIGURE 3. Gonadal-somatic index of prenatal stressed and control offsprings from LNL and HNL groups. The results are expressed as mean ± SEM, with an n = 8/bar. * p < 0.05: Stress vs. Control. * * p < 0.05: Control LNL vs. Control HNL at 45 days,. * * * p < 0.05: Control, LNL vs. Control HNL at 70 days.
In addition, prenatally stressed male offspring belonging to LNL had a smaller gonadal-somatic index than controls at 45 and 70 days of age; differences in HNL were not significant.
The ANOVA of TES levels revealed significant effects of offspring number (F (1,85) = 16.31, p = 0.001); treatment (F (1,84) = 57.34, p = 0.001) and time (F (2,84) = 588.72, p = 0.001), as well as significant interaction between the three factors (F (2,84) = 56.76, p. = 0.001).
Plasma TES levels in control male LNL offsprings were higher at 70 days of age than the concentrations observed in control HNL animals. However, the TES concentrations in stressed LNL offsprings at 70 days of age were significantly lower than the concentrations in stressed HNL offsprings (Fig. 4).

FIGURE 4. Plasma testosterone levels in LNL and HNL groups. The results are expressed as mean ± SEM, with a n = 8/bar. 8* p < 0.05: Stressed vs. Control * * p < 0.05: Control LNL vs. Control HNL at 70 days. * * * p < 0.05: Stress LNL vs. Stress HNL at 70 days.
On the other hand, when comparing the levels of TES between treatments within a litter we observed that the plasmatic levels of TES were significantly lower in the stressed animals in comparison with their controls at 70 days of age in LNL groups. The same tendency was observed in HNL offsprings, however, differences were not significant (Fig. 4).
The ANOVA test indicated a significant effect on LH levels of the treatment (F(1,49) = 11.83, p = 0.0011), as well as an interaction between treatment and time (F(1,49) = 4.35, p = 0.042).
When analyzing plasma LH levels in 70 days old control animals from both litters, it was observed that LH levels were higher in HNL than in LNL (Fig. 5). However, LH levels of stressed animals from both litters were similar. Stress had a significant effect on LH concentrations in HNL animals.

FIGURE 5: Plasma LH levels (mg/dl) in prenatally stressed and control offsprings from LNL and HNL groups. The results are expressed as mean ± SEM, n = 13/bar for LNL and HNL stressed animals. n=12 and n=15 for LNL and HNL controls, respectively. * p < 0.05: Stressed vs. Control at 70 days. * * p < 0.05: Control LNL vs. Control HNL at 70 days.
Discussion
Our results show that the corporal weight was not significantly affected by prenatal stress. Prenatal stress increased the adrenal-somatic index significantly only in male LNL offsprings. The higher adrenal-somatic index in LNL animals could be due to the fact that these animals probably had higher adrenal gland weight at the moment of birth, as a consequence of higher levels of maternal ACTH. The decrease in serum progesterone levels, which in gestation are proportional to the number of fetuses, could be the cause of the increase of hypothalamic-pituitary-adrenal axis sensitivity, with higher secretion of ACTH during stress. (Viau and Meaney, 1991; Sugino et al., 1994). Sugino et al. (1991) suggested that the corpora lutea of pregnant rats with more than ten conceptus, which were sufficiently exposed to placental hormones before day 12 of pregnancy may not be influenced by the pituitary-adrenal system. In addition progesterone may inhibit ACTH secretion during stress (Viau and Meaney, 1991). The higher concentration of serum progesterone in HNL than in LNL groups may block the effect of stress by inhibiting ACTH release from the pituitary.
This effect could persist during the postnatal life, without necessarily implying hormonal differences, generating a probable morfofunctional dissociation. This seems to happen in the male offsprings of smaller litters.
These results agree with those observed in adult animals exposed to repeated social or immobilizations stress, which induces an increase of the weight of the adrenal gland (Lemaire et al., 1997; Martí et al., 1994) that persists through time without generating morpho-functional dissociation.
This could also explain the absence of significant differences in the adrenal-somatic index between control and stressed HNL offsprings, because the stressed mothers' axis could be desensitized (lower levels of ACTH and COR) due to the protective effect of gestation hormone levels (Viau and Meaney, 1991; Sugino et al., 1994). Significant differences were also observed in plasma COR levels in prenatally stressed LNL animals.
These results suggest that the hypothalamic-pituitary-adrenal axis of prenatal stress rats is hyperactive, as the levels of basal plasmatic COR are higher than their corresponding controls. These findings agree with those of Clarke et al. (1994), Fride et al. (1986) and Peters (1982). The hyperactivity observed was manifested with an increase of the total size of the adrenal gland in prenatally stressed LNL animals. However, only a tendency was observed in HNL males at the same age. This result could be due to the protective effect of high levels of maternal progesterone (Viau and Meaney, 1991). Those results are consistent with those from Weinstock (1997) that indicated that prenatally stressed male offspring did not show an increase of the basal COR levels.
The effects of pregnant female stress on the hypo-thalamic-pituitary-adrenal offsprings axis are possible since the maternal COR crosses the placenta and reaches the fetus, probably affecting adrenal development and generating a stronger cortex-marrow relationship, as observed by Mayer (2004) and Klemcke et al. (1995).
The hyperactivity of the hypothalamic-pituitary-adrenal axis may have been generated by the decrease in the number of COR receptors present in the hippocampus of the offsprings (Maccari et al., 1995; Maccari et al., 2003), which are the main control of COR adrenal secretion by negative feedback. Thus, a decrease of these corticosteroid receptors would be followed by an increase of COR secretion.
With regard to the gonadal-somatic index, it was observed that the prenatally stressed male offspring belonging to LNL had a lower gonadal-somatic index than their controls at 45 and 70 days of age. There is evidence that prenatal stress reduces testicular weight in recently born males (Dahlof et al., 1978) similar results were observed in males of 105 and 115 days of age (Meisel et al., 1979; McLeod and Brown, 1988). In the HNL groups, differences between stressed animals and their controls were not significant.
Plasmatic TES levels showed a clear reduction in stressed LNL males of 70 days of age. However, only a tendency was observed in the HNL males of the same age. Other authors have reported similar results (Gerardin et al., 2005). In control animals, the plasmatic TES values in 70-day-old LNL rats were higher than those in HNL males.
The number of offsprings per litter might affect the TES values in control animals. When comparing the TES values with the gonadal-somatic index, a relationship was observed between the gonad size and hormone levels, it is well known that the TES concentrations are intimately related with the deg ree of testicular developmen. The same considerations that explain differences in gonadal-somatic index between litters can be applied to TES data. A additional stress during the last time of gestation might be a possible explanation (Ward et al., 2003; Marques Pereira et al., 2006).
It is known that fetuses exposed to environmental factors or pharmacological agents suffer alterations that inhibit the normal gonadal activity, which in turn affects sexual development and behavior in adulthood (Taché et al., 1980; Ward et al., 1994). Also is known that chronic immobilizations applied in mature males produces a drastic fall of the levels of plasma TES until 18 hs after stress (Tsuchiya and Horrii, 1995). This may explain the results, related to the gonadal-somatic index and the testicular TES production in LNL offsprings. HNL animals showed the same the tendency.
These are few studies regarding the mechanisms through which the prenatal stress exercises its action on TES secretion. It is known that administration of pharmacological doses of catecholamines reduces the plasmatic TES levels in male rats (Damber and Janson, 1978; Levin et al., 1967; Stahl et al., 1984), while these levels increase after adrenalectomy (Frankel and Ryan, 1981). On the other hand, perinatal administration of antagonists of the GABAergic receptors, such as picrotoxina, in the dorsomedial hypothalamus, which induces an increase of the plasma COR and hypothalamic noradrenergic, produces a decrease of the plasma TES in mature animals (Silva et al., 1998). Therefore, it is possible that stress exercises its effect in part, through this mechanism.
Charpenet et al. (1981) demonstrated that Sertoli cells express β2-adrenergic receptors and that, through these receptors, catecholamines stimulates estradiol secretion. High estradiol levels act on Leydig cells and inhibit TES production. Although this mechanism has been described only in mature animals, it is likely that a similar process takes place during fetal development at the mother's catecholamines expense. Similar results were reported by Ward et al. (2003). This might probably produce a lower liberation of TES in adult life as a result of a modification of the gonad functionality.
Differences observed in the levels of LH plasma in control animals of 70 days of age could be due to a modification in the negative feedback mechanism of TES on hypothalamic-pituitary-gonadal axis. Indeed, in LNL animals, plasma TES levels were elevated in postnatal life, and this could inhibit the hypothalamic-pitu-itary-gonadal axis activity, producing a significant decrease in LH secretion. In the HNL rats, as TES levels at 70 days of age were lower, the inhibitory effect on the hypothalamic-pituitary-gonadal axis could be lower, which might explain the higher plasma LH levels.
When analyzing the plasma LH levels in stressed males, we observed a significant decrease in the HNL groups at 70 days of age. In LNL, there were no significant differences.
Stress effects on the hypothalamic-pituitary-gonadal axis hormones are relatively complex. It is known that stress stimuli of short duration, independently of their intensity, cause an increase in LH secretion, while LH levels decrease when the stimuli last several hours and are of high intensity (Lopez-Calderon et al., 1990; Silva et al., 1998). Some studies attempted to elucidate the mechanisms implied in the inhibition of LH secretion by longer and more severe stress situations. The role of the monoamines is not known and the experimental data point to an inhibitor effect of the CRH, acting directly or indirectly through the opiate endogenous activation that play a predominantly inhibiting role in the gonadotropins secretion (Rivier and Rivest, 1991; Rivier and Vale, 1986).
In conclusion, maternal COR seems to affect the hypothalamic-pituitary-gonadal axis of the fetus diminishing the testicular development represented by the gonadal-somatic index. As consequence, Leydig cells TES production is affected in LNL animals when reaching sexual maturity. A another probable mechanism would be mediated by catecholamines, inhibiting TES synthesis through the adrenergic receptors present in Sertoli cells.
Effects of prenatal stress in the LNL rats are manifested mainly at the gonadal level without apparent modifications at pituitary level, no changes were observed in offsprings LH levels.
On the contrary, in the HNL animals the impact of stress might be specifically at pituitary level. The absence of changes in the parameters measured at gonadal level in these litters could have at least two explanations. On the one hand, the effects of stress at gonadal level seem to have a lower sensitivity in HNL animals after both litter's comparisons. In this respect, it is well known that there is a direct relationship between the number of fetuses in the uterus of pregnant rats and circulating progesterone levels (Kato et al., 1979), could enhance the inhibitory effect on the sensibility of the mother's hypothalamic-pituitary-adrenal axis, exercising a lower stress effect on the litter. These antecedents indicate that the rats belonging to litters with a high number of offsprings might be more resistant to stress than those belonging to litters with few offsprings.
On the other hand, it is probable that the effects of stress were not manifested in HNL animals in the measured times, since it seems to exist a certain delay in the gonad growth sexual development, both in control and experimental animals. This might produce a weaker negative feedback of the hypothalamic-pituitary-gonadal axis.Our data suggest that HNL animals are more sensitive to the effects of prenatal stress evaluated through the plasmatic LH levels. However, the absence of significant differences in the LH levels between stressed and control animals in LNL suggest that stress affects the levels of LH differently depending on litter size. The effect of stress on LH levels in LNL rats could be masked by the negative feedback that TES exerts on the HPG axis.
References
1. Closet A, Perelló A, Lopez Calderón A (1986). Adrenocorticotropin Administration increases Testosterone Secretion in Adult Male Rats. Life sciences 39: 1119-1122. [ Links ]
2. Chantal H, Arsaut J, Arnauld E, Demotes-Mainard J (1996). Transient neonatal elevation im hypothalamic estrogen receiving mRNA in prenatally-stressed male rats. Neuroscience Letters 216: 141-145. [ Links ]
3. Charpenet G, Crossout Y, Forest MG, Haour F, Saez JM, Bernier M (1981). To take a shower, J. R. Collu, R. Effects of chronic Intermittent Immobilization Stress on Rat Testicular Adrogenic Funtion. Endocrinology 109(4): 1254-1258. [ Links ]
4. Charpenet GI, Crossed Y, Bernier M, Ducharme, J, Collu R (1982). Stress-Induced Testicular Hyposensitivity to Gonadotropin in Rats. Role of the Pituitary Gland. Biol Reprod. 27: 616-623. [ Links ]
5. Chrousos G, Loriaus D, Gold P (1988). Machanisms of phisicals and emotional stress. In: Advances in experimental medicine and bilogy. Chrousos G, Loriaus D and Gold P, Eds. Plenum Press, New York and London pp. 1-77. [ Links ]
6. Clarke AS, Wittwer DJ, Abbott DH, Scheneider ML (1994). Long-Term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 27: 257-269. [ Links ]
7. Collu P, Gibb W, Ducharme JR, Singer GA (1984). Role of the catecholamine in the inhibitory effect of immobilization stress on testosterone secretion in rats. Bio Reprod. 30: 416-422. [ Links ]
8. Dahlof LG, Hard E, Larsson K (1978). Influence of maternal stress on the development of the fetal genital system. Physiol Behav. 20: 193-195. [ Links ]
9. Damber JE, Janson PO (1978). The effects of LH, adrenaline and noradrenaline on testicular blood flow and captures testosterone concentrations in anaesthetized rats. Records Encrinol. (Copenh) 88: 390-396. [ Links ]
10. Fisher LA, Browm MR (1991). Central regulation of stress responses: regulation of the autonomic nervous system and visceral function by corticotrophin releasing factor-41 Baillere's Clim. Endocr Metab. 5(1): 35-50. [ Links ]
11. Frankel AI, Ryan EL (1981). Testicular innervations is necessary for the response of captures testosterone levels to acute stress. Biol Reprod. 24: 491-495. [ Links ]
12. Fride E, Weinstock M (1984). The Effect Prenatal of Exposure to Predictable or Unpredictable Stress on Early Development in the Rat. Dev Psychobiol. 17(6): 651-660. [ Links ]
13. Fride E, DanY, Feldon J, Halevy G, Weinstock M (1986). Effects of prenatal stress on vulnerability to stress in prepuberal and adult rats. Physiol Behav. 37: 681-687. [ Links ]
14. Gerardin DCC, Pereira OCM, Kempinas WG, Florio JC, Moreira EG, Bernardi MM (2005). Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav. 84: 97-104. [ Links ]
15. Kato H, Morishige KW, Rothchild LA (1979). Quantitative relation between the experimentally determined number of conceptuses and corpus luteum activity in the pregnant rat. Endocrinology. 105(3): 846-850. [ Links ]
16. Kinsley C, Svare B (1986). Prenatal stress effect: does it plow they mediated by reductions in maternal food and water intake and body weight gain? Physiol Behav. 37: 191-193. [ Links ]
17. Klemcke H (1995). Placental metabolism of cortisol at mid-and late gestation of swine. Biol Reprod. 3: 1293-1301. [ Links ]
18. Krey L, Lu K, Butter W, Hotchkiss J, Piva F, Knobil E (1975). Surgical disconnection of medical basal hypothalamus and pituitary in the rhesus monkey. II. GH and cortisol secretion. Endocrinology 102: 142-150. [ Links ]
19. Lemaire V, Taylor GT, Mormède P (1997). Adrenal axis activation by chronic social stress fails to inhibit gonadal function in male rats. Psychoneuroendocrinology 22(8): 563-573. [ Links ]
20. Levin J, Lloyd CW, Lobotsky J, Friedich EH (1967). The effects you epinephrine on testosterone production. Records Endocrinol (Copenh) 55: 184-192. [ Links ]
21. López-Calderon A, Gónzalez-Quijano MI, Tresguerres JAF, Ariznavarreta C (1990). Role of LHRH in the gonadotrophin response to restraint stress in intact male rats. J Endocrinol. 124: 24. [ Links ]
22. Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M (1995). Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 15: 110-116. [ Links ]
23. Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003). Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 27: 119-127. [ Links ]
24. Marques Pereira OC, Bernardi MM, Ceccatto Gerardin DC (2006). Could neonatal testosterone replacement prevent alterations induced by prenatal stress in male rats? Life Sci. 78: 2767-2771. [ Links ]
25. Martí O, Martí J, Closet A (1994). Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 55: 747-753. [ Links ]
26. Mayer NI (2004). Effects of prenatal stress on male offspring neuroendocrine and immunologic parameters. Doctoral thesis pp. 1-90. [ Links ]
27. McLeod PJ, Brown RE (1988). The effects of prenatal stress and postweaning housing conditions on parental and sexual behavior of male Long-Evans rats. Psichobioly 16(4): 372-380. [ Links ]
28. Meisel RL, Dohanich GP, Ward IL (1979). Effects of prenatal stress on avoindance acquisition, open-field performance and lordotic behavior in male rats. Physiol Behav. 22: 527-530. [ Links ]
29. Menéndez-Patterson A, Fernandez F, Marin B (1982). In uterus, immobilization stress and its effects on the development, behavior and sexual maturity of the rat. Revista Española de Fisiología. 38: 433-440. [ Links ]
30. Michajlovskij N, Lichardus B, Kvetñasnsky R, Ponec J (1988). Effect of acute and repeated immobilization stress on for and water intake urine output and vasopressine changes in rats. Endocrinologia Experimentalis 22: 143-157. [ Links ]
31. Neumann I, Wigger A, Liebsch G, Holsboer F, Landgraf R (1998). Increased basal activity of the hypothalamo-pituitary-adrenal axis during pregnancy in rats bred for high anxiety in related behavior. Psychoneuroendocrinology 23(5): 449-463. [ Links ]
32. Orr TE, Mann DR (1990). Effects of restraint stress on LH and testosterone concentrations, Leydig cell LH/HCG receptors, captures and in vitro testicular steroidogenesis in adult rats. Horm Behav. 24: 324. [ Links ]
33. Peters DAV (1982). Prenatal stress: Effects on brain biogenic amines and plasma corticosterone levels. Pharmacol Biochem Behav 17: 721-726. [ Links ]
34. Rivier C, Vale W (1986). Stress-induced inhibition of reproductive functions role of endogenous conticotropin-releasin factor. Life Sci. 23: 607-609. [ Links ]
35. Rivier C, Rivest T (1991). Effect of stress on the activity of the hypothalamic-pituitary-Gonadal axis: peripheral and central mechanisms. Biol Reprod. 45: 523-526. [ Links ]
36. Silva MRP, Olivera CA, Felicio LF, Nasello AG, Bernardi MM (1998). Perinatal treatment with picrotoxin induces sexual, behavior, and neuroendocrine changes in male rats. Pharmacol. Biochem Behav. 60(1): 203-208. [ Links ]
37. Stahl F, Götz F, Dörner G (1984). It captures testosterone level in rats under varius conditions. Exp Clin Endocrinol. 84: 277-284. [ Links ]
38. Sugino N, Tanura H, Nakamura Y, Ueda K, Kato H (1991). Differential mechanisms for the inhibitions of progesterone secretion by ACTH and corticosterone in pregnant rats. J Endocrinol. 129: 405-410. [ Links ]
39. Sugino N, Nakamura Y, Okuno N, Shimamura K, Tellama T, Ishimatsu M, Kato H (1994). Effects of restraint stress on luteal function in rats during mid-pregnancy. J Reprod Fertil. 101: 23-26. [ Links ]
40. Tache Y, Ducharme JR, Charpenet G, Haour F, Saez J, Collu R (1980). Effect of chronic intermittent immobilization stress on hypophyso-gonadal function of rat. Records Endocr. Copenh. 93: 168-174. [ Links ]
41. Tsuchiya T, Horrii I (1995). Different effects of acute and chronic immobilization stress on plasma testosterone levels in male syriam hamsters . Psychoneuroendocrinology 20(1): 95-102. [ Links ]
42. Viau V, Meaney MJ (1991). Variation on the hypothalamic pituitary adrenal response to stress during the estrous cycle in the rat. Endocrinology 129: 2503-2511. [ Links ]
43. Ward IL, Weisz J (1980). Maternal stress alters captures testosterone bad in fetal. Science 207: 328-329. [ Links ]
44. Ward IL, Weisz J (1984). Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male female rat fetuses and their mothers. Endocrinology. 114(5): 1635-1644. [ Links ]
45. Ward IL, Ward OB, Winn RJ, Bielawski D (1994). Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress or both factors. Behav Neurosci. 108: 1188-1195. [ Links ]
46. Ward IL, Ward OB, Afusso JD, Long III WD, French JA, Hendricks SE (2003). Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav. 43: 531-539. [ Links ]
47. Weinstock M (1997). Does prenatal stress impair copinf and regulation of hypothalamic-pituitary -adrenal axis? Neurosci Biobehav Reviews. 21(1): 1-10. [ Links ]
48. Weisz J, Ward L (1980). Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 106: 306-316. [ Links ]
Received on July 27, 2006.
Accepted on December 19, 2006.














