Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.31 no.3 Mendoza Sept./Dec. 2007
Effects of desiccation on Euterpe edulis Martius seeds
Víctor Panza1,2, Verónica Láinez2, Sara Maldonado1,2, And Horacio L. Maroder3
1. Instituto de Recursos Biológicos CIRN, INTA. Las Cabañas y Los Reseros s/n. B1712WAA, Hurlingham. Buenos Aires, Argentina.
2. Departamento de Biodiversidad y Biología Experimental. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Pabellón 2, Ciudad Universitaria, C1428EHA, Buenos Aires, Argentina.
3. Departamento de Ciencias Básicas-Universidad Nacional de Luján. Ruta 7 y 5, (6700). Luján, Provincia de Buenos Aires, Argentina.
Address correspondence to: Dr. Sara Maldonado. Departamento de Biodiversidad y Biología Experimental. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Pabellón 2, Ciudad Universitaria. C1428EHA, Ciudad Autónoma de Buenos Aires, ARGENTINA. E-mail: saram@bg.fcen.uba.ar
ABSTRACT: Information on desiccation sensitivity of Euterpe edulis seeds under two drying rates is presented. The sensitivity was studied during the course of germination and normal germination. The water content was evaluated for both seeds and embryos. Results showed the following: (a) For both drying treatments and for both germination and normal germination, desiccation sensitivity values were higher for measurements based on the water content of the embryo than for those of the seed. (b) For both drying treatments, desiccation sensitivity were higher for normal germination than for germination based on both the embryo and seed water contents. (c) Under the slow drying treatment and for measurements based on the seed water content, critical water content was visible for normal germination but not for germination; (d) Critical water contents for germination and normal germination were more clearly established in the fast drying treatment than they were in the slow drying method based on both the embryo and seed water contents. Critical water contents were not associated with changes in electrolyte leakage, which suggests that conductivity is not a good indicator of physiological seed quality. From the beginning of both drying treatments, changes in nuclei and vacuoles were observed, but, when seed water content was reduced to below critical values, the cells became severely plasmolyzed, the vacuoles highly distorted, and the nuclei formed an almost homogeneous mass with the chromatin and the nucleoplasm, which suggests irreversible DNA damages.
Key words: Drying chamber dehydration; Embryo desiccation sensitivity; Euterpe edulis seed; Silica gel drying; Recalcitrant seed.Introduction
Euterpe edulis exhibits recalcitrant seed-storage behaviour; in other words, seed viability begins to decrease at approximately 39% seed water content and is definitively lost at 21% water content (Andrade, 2001; Martins et al., 2000; Reis et al., 1999). In addition, Panza et al. (2004) report subcellular recalcitrant features of the E. edulis embryo tissues, i.e., cells highly vacuolated (at high water content), almost lacking storage reserves, with abundant endomembranes, and abundant ergastic substances. The differential water content between the embryo axis (and embryo) and the storage tissue presents a difficulty for interpreting the experimental results on recalcitrant seeds (Berjak et al., 1989). This difficulty is exacerbated when the embryonic axis constitutes a small fraction of the total volume of the seed, and its water content is considerably higher than that of the storage tissues (ISTA, 1989), which is the situation with E. edulis seeds. A preliminary study indicates that the whole embryo constitutes only 0.54% of the seed fresh weight with 85% water content, while that of the endosperm is 48.2% (Panza et al., 2001).
When submitted to dehydration, recalcitrant seeds show variable critical values according to the speed of dehydration (Farrant et al., 1985; Pritchard, 1991). However, there are conflicting opinions on this subject (Bonner, 1996; Finch-Savage, 1992; Pritchard et al., 1995).
Transmission electron microscopy shows that recalcitrant seeds exhibit a progressive degradation of membranes during drying (Pammenter and Berjak, 1999). In the recalcitrant seeds of Acer saccharinum L. and Aquilaria agallocha Roxb, damages in cell membranes increase solutes leakage and, consequently, conductivity (Beewar et al., 1982; Kundu and Kachari, 2000). Although leakage has been correlated with the loss of seed viability and germination, such correlation has been scarcely investigated in recalcitrant seeds.
In orthodox seeds, during the desiccation-tolerant phase, the chromatin is in a highly condensed state while the seeds are desiccation-tolerant. However, this is clearly a reversible process and rehydration to the state of resumption of DNA replication is associated with chromatin decondensation (Pammenter and Berjak, 1999). Chromatin compaction is also observed in recalcitrant seeds with increasing water loss, although here the compaction is irreversible (Pammenter and Berjak, 1999). This suggests that the desiccation-sensitive embryo tissues of recalcitrant seeds lack the orderly and reversible mechanism that provide stability to chromatin in the desiccation-tolerant embryo tissues of orthodox seeds.
The present study examined the germination course in function of the water content of the whole seed and the embryo at two drying rates. Subcellular analysis by transmission electron microscopy (TEM) and electrolyte leakage by conductivity tests were also used to evaluate the dehydration process. To obtain exact knowledge of the desiccation sensitivity of these seeds, we determined the embryo water content at which germination parameters are affected; this information was previously unavailable.
Material and Methods
Mature fruits of E. edulis were harvested in the Parque Nacional Iguazú, province of Misiones, Argentina (S25º40'50.4'' W54º09'24.0) during the month of August for three consecutive years, from 1999 to 2001, and shipped by expedited post to the Germplasm Bank of INTA, Castelar, Buenos Aires, Argentina, where the experiments were conducted.
Fruits were stored in 30 mm-thick polyethylene bags at 4°C. At this temperature, seeds did not show sensitivity to chilling. During the first days of storage, pericarps were removed. Seeds were carefully selected to assure that the samples were similar in size and germination percentage.
Desiccation methods
Two drying methods were used. In both, seeds were maintained in a single layer in plastic mesh bags. Rapid drying was carried out by placing the bags inside silica gel (approximately 2% RH, Vaisala humidity and temperature indicator HMI 31 Finland) contained in desiccators at 25 ± 1°C. The silica gel/seed ratio was 8/1 (w/ w) and silica gel was replenished daily. Seed samples were removed from the desiccators at 24, 48, 72, 96, and 144 h. Slow drying was carried out by placing the bags on a metal mesh in a drying chamber at 21 ± 2% RH and 19 ± 2ºC. Samples were removed periodically during 17 days. Seeds from both drying treatments were used for germination testing, conductivity testing, and light and transmission electron microscopy. The water contents (percentage wet weight basis) of the whole seed and the isolated embryos were determined on three replications of five seeds by the ISTA (2005) oven methods (103ºC ± 1ºC for 17 h).
Germination tests
Germination tests were performed in plastic boxes on moist germination paper and cotton at 25 ± 1°C, 16 h fluorescent light (16 mmol m-2 s-1). Initially, germination was assessed three times per week. Each test used 3 x 20 seeds. Germination and normal germination were evaluated. Germination corresponds to button protrusion, i.e., emergence of cotyledon tissue forming a wheel around the hole from which the radicle protrudes. Normal germination corresponds to the plantule having its first three leaves (the third leaf being the first one with pinnate structure) and a welldeveloped radicle (Belin-Depoux and Hering de Queiroz, 1971). Evaluation of normal germination was accessed at weekly intervals during 25 weeks. The critical water contents of the seed and embryo were taken at the point in which the germination significantly decreased.
Transmission electron microscopy
Embryos isolated from seeds of both control and silica gel drying treatment were immediately fixed. In all cases, root and shoot apexes were excised and fixed overnight at 4°C in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2). Tissues were post-fixed in 1% OsO4 in water for 2 h, and then they were dehydrated in a graded ethanol-acetone series and embedded in low viscosity Spurr's resin. Once germination curves were known, shoot and root apical meristems of those seeds corresponding to: (I) control, (II) initiation of germination decrease, and (III) remarkable germination decrease, were further processed for transmission electron microscopy (five samples for each point). For transmission electron microscopy, ultra-thin sections were mounted on grids, stained with uranyl acetate followed by lead citrate (Maldonado and Lott, 1991), and examined in a Zeiss EM109T microscope.
Conductivity tests
Electrolyte leakage from the embryos was determined by placing six embryos in 10 ml of distilled water at 15°C. Conductivity was measured after 3 h of soaking. Results were expressed as mS mg-1 of dry embryo tissue. Three replications were carried out. Values corresponded to mean ± SD. To avoid damage of imbibitions, embryos were placed for three hours in 100% relative humidity (over water) before immersion in distilled water.
Statistical analysis
To determine the critical water content values, percentage data for germination and normal germination were transformed in arcsin Ö(x/100) and subjected to analysis of variance and comparison of the means by Tukey's test at a = 0.05.
Results
Shortly after shedding, the germination percentage was 97.5%. The mean water contents were 85.3% for isolated embryos and 48.2% for whole seeds (wet weights).
Drying rates of both embryos and whole seeds were higher in the silica gel (fast drying) treatment than in the drying chamber (slow drying treatment) (Fig. 1). After 6 days of the silica gel treatment, water content decreased to 44.9% for embryos and 29.7% for whole seeds, while the corresponding values for the drying chamber treatment were 54% and 37% (Fig. 1).

FIGURE 1. Drying curves of E. edulis seeds dried with silica gel (25ºC and 2% R.H.) and drying chamber (19ºC and 21% R.H.). For both treatments, the rates of water loss are represented by a second order polynomial equation for embryo and a linear equation for whole seed. Symbols: () embryo after silica gel treatment, () embryo after drying chamber treatment, () endosperm after silica gel treatment, () endosperm after drying chamber treatment, () seed after silica gel treatment, (❍) seed after drying chamber treatment, ___ polynomial equation, --lineal equation. Vertical bars represent SD of the means.
Under the silica gel treatment, the germination percentage gradually declined to 80% (Figs. 2A and B) when the water content was decreased to 48.5% (embryo) and 35.9% (whole seed). Under the silica gel treatment, the germination percentage gradually declined to 80% (Figs. 2A and B) when the water content was decreased to 48.5% (embryo) and 35.9% (whole seed). The germination percentage dropped to 22% with slightly more drying: 3.6% in the embryo and 8.2% in the whole seed, in regards to water content. Consequently, the values of 48.5% (embryo) and 35.9% (whole seed) water content were taken to be the critical water contents.
Under the drying chamber treatment, the critical water content for germination was not visible (Figs. 2C and D). However, statistical analysis indicated that the narrow interval between 43 and 48.8% water content could be considered as the critical water content for embryos (Fig. 2C).
Normal germination was always lower than germination for the same water content (Figs. 2A-D). Under the silica gel treatment, normal germination showed a slight increase to 81% that was not statistically significant when water content decreased to 60.6% (embryo) and 40.5% (whole seed). At 55% (embryo) and 38% (whole seed) water content, normal germination markedly decreased, and, therefore, those values were considered as critical water contents (Figs. 2A and B). Under the drying chamber treatment, critical water contents for normal germination were 67% (embryo) and 41.7% (whole seed) (Figs. 2C and D).
For normal germination, critical water contents for both embryos and whole seeds were higher for the drying chamber treatment than for the silica gel treatment, i.e., the susceptibility of normal germination water loss was dependent on the drying rate. Therefore, unlike under slow drying, under fast drying the seed can reach a lower water content without affecting normal germination. For germination, comparisons between the critical water contents at the two drying rates could be established only for the embryo water content (Figs. 2A and C). Comparison could not be established for whole seed water content because no critical water content value was observed for drying chamber treatments (Fig. 2D).
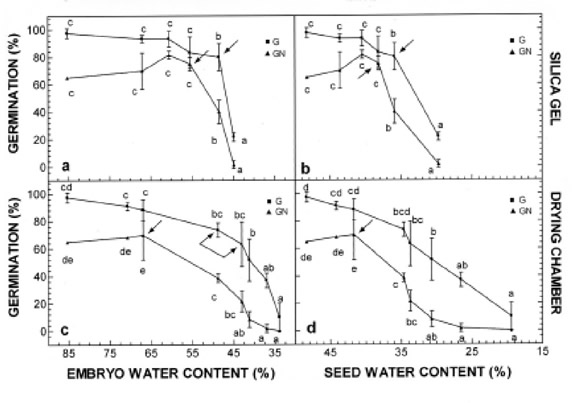
FIGURE 2. Germination curves of E. edulis seeds dried by two different methods. A and B: Seeds dried with silica gel at 25ºC and 2% R.H. Germination (G) and normal germination (NG) are expressed in function of A) embryo water content, and B) seed water content, C and D: Seeds dried in drying chamber at 19ºC and 21% R.H. Germination (G) and normal germination (NG) are expressed in function of C) embryo water content, and D) seed water content. Vertical bars represent sd of the mean. Different letters indicate significance as calculated by Tukey's test at <0.05. In all curves, an arrow indicates critical water content. In C, an interval between 48.8 and 43% water content is considered as critical for the embryo.
Embryo critical water content resulted in similar values for both drying treatments, which suggests that germination is dependent on the water content and not on the drying rate.
When seeds were dried by the silica gel treatment and germination had declined to 80%, cells of the apical meristems showed structural changes indicative of some deterioration (Figs. 3A-D). Compared with freshly harvested seeds, the dried seeds had chromatin that was more compacted, and, consequently, the nucleoli were difficult to distinguish. Some vacuoles became confluent and formed larger compartments (Fig. 3D). Most cells maintained their shape, but some cell walls appeared to be less rigid. The confluence of lipid bodies was characteristic. Also, there was localized withdrawal of the plasmalemma from the cell wall where some vesicles had been extruded.
When seed water content was reduced to below critical values and loss of germination was observed, the general structure of all embryo tissues showed evidence of considerable damage (Figs. 3E and F). The chromatin and the nucleoplasm formed an almost homogeneous mass. Cells were severely plasmolyzed and extra-plasmalemma vesicles, indicative of exocytosis, were visible (Fig. 3F). Vacuoles were reduced in the contracted cytoplasm.

FIGURE 3. Light and Transmission Electron Microscopy of embryo tissues. A and B: Sections obtained from mature E. edulis embryos immediately following harvest. A: Shoot apical meristem (in the button) and cotyledon (co). B: An initial cell from shoot apical meristem. Numerous mitochondria, several small vacuoles, and a large nucleus can be observed. C and D: Sections obtained from E. edulis embryo tissues after drying with the silica gel method. Germination had declined from 97% to 80% (48.5% embryo water content and 35.9% whole seed water content). C: Shoot apical meristem and cotyledon. D: An initial cell from the shoot apical meristem showing changes in the chromatin. Vacuoles become confluent and formed larger compartments. E and F: Sections obtained from E. edulisembryo tissues after drying with the silica gel method. Seed water content had been reduced to critical values (60.6% and 40.5% water content for embryo and whole seed respectively) and almost total loss of germination had occurred. E: Shoot apical meristem. Nuclear contents can be observed as an almost homogeneous mass. Arrows indicate the border of the shoot apical meristem. F: Detail of an initial cell from the shoot apical meristem showing severe plasmolysis, secretion of extraprotoplasmic vesicles (black arrow), and reduction of vacuolar volume in the contracted cytoplasm; chromatin and nucleoplasm constitute an undifferentiated mass. In all figures N: nucleus, V: vacuoles and C: chromatin. For A, C, and E, bars = 20 mm; for B, D, and F, bars = 2 mm.
Electrolyte leakage from embryos progressively increased with desiccation, particularly at low water contents, which suggested membrane deterioration (Figs. 4A and B). The critical water contents were not associated with changes in electrolyte leakage.

FIGURE 4. A and B: Electrolyte leakage of E. edulis embryo tissues during desiccation to different water contents. A: in seeds dried with silica gel at 25ºC and 2% R.H. B: dried in drying chamber at 19ºC and 21% R.H. In both cases, the rate of water loss could be described by an exponential equation.
Discussion
This study compared the susceptibility to desiccation of embryos to that of whole seeds. Berjak et al. (1989) describe the difficulty of comparing water contents when embryo or whole seed are measured. We found that such difficulty was exacerbated in this species because the embryo constitutes only 0.54% of the seed fresh weight. In addition, dehydration curves showed that water loss was faster for the embryo than for the endosperm.
Queiroz et al. (1986) indicate that during germination of fresh E. edulis seeds, the percentage of button protrusion is high and positively correlated with plumule emergence. However, we observed that the development of a seedling with three leaves did not always follow button protrusion, and some death could occur after plumule emergence. A plantule with three leaves seems to be the most appropriate growth stage for germination evaluation; i.e., normal germination was lower than germination at most embryo and whole seed water contents. Consequently, two germination stages were evaluated: 1) button protrusion (germination) and 2) a subsequent stage represented by the plumule emergence, i.e., seedlings exhibiting both a well-developed radicle and a shoot with the first three leaves, the third leaf being the first one with pinnate structure (normal germination). Using both germination stages we found the following results: (a) For both drying treatments, desiccation sensitivity values were higher for both germination and normal germination based on the embryo water content, i.e., the embryo desiccation sensitivity was masked for measurements based on the whole seed water content. This result demonstrates the convenience of basing evaluations on both the embryo and the whole seed water contents to obtain a better knowledge of the desiccation sensitivity of these seeds. (b) For both drying treatments, desiccation sensitivity was higher for normal germination than for germination based on both the embryo and the whole seed water contents; therefore, a reliable evaluation of drying effects on germination should include the stage with the plantule with three leaves. (c) Under the slow drying treatment and based on the whole seed water content, critical water content was visible for normal germination and not for germination; therefore, germination was not appropriate for detecting critical water content based on the whole seed water content. (d) Critical water contents for germination were most clearly established in the silica gel treatment based on both the embryo and whole seed water contents. Under the drying chamber treatment, critical water contents were only established for normal germination based on both the embryo and the whole seed water contents. (e) For both drying treatments, critical water content was higher for normal germination than for germination. (f) For the drying chamber treatment, when germination was based on the whole seed water content, critical water content was not visible.
Some studies report that critical values for recalcitrant seeds are subject to variation according to the speed of dehydration (Farrant et al., 1985; Pritchard, 1991). However, there are conflicting opinions on this subject (Bonner, 1996; Finch-Savage, 1992; Pritchard et al., 1995). For E. edulis seeds, we found that the critical water content for normal germination depended on the drying method, but that for germination did not. Under the drying treatments used here, critical points for the viability of the E. edulis embryo could be more precisely determined when the germination parameters were evaluated in function of the water content of the embryo, but not of the whole seed. These results highlight the need to evaluate the water content based on the embryo.
Queiroz et al. (1986) found that, when E. edulis seeds with initial water content of 51.3% are slightly dried, their germination percentage significantly increases from 81% to 98%. We used seeds with an initial water content of 48.5% and observed a slight increase in the germination percentage only for normal germination (based on the embryo water content) at initial drying; however, the increase was not statistically significant. We suggest that, for newly collected seeds that differ in water content and physiological state (as those supposedly used by Queiroz et al., 1986), the initial germination percentage could be increased by slight desiccation.
As indicated by the progressive increase in electrolyte leakage and by the transmission electron microscopic images, cell membrane damages occurred especially at high desiccation levels. Critical water contents could not be associated with any abrupt change in the conductivity curve, which suggests that conductivity is not an appropriate method for detecting the critical water content in this species.
TEM images of shoot apical tissues of the E. edulis axis show that, in mature seeds, chromatin is present in a natural incipient condensed state, which might be related to the relative quiescence exhibited by the apical meristems (Panza et al., 2004). As in orthodox seeds, this is a reversible state where the rehydration is associated with decondensation of chromatin (Pammenter and Berjak, 1999). During E. edulis seed dehydration, gradual intracellular injury of organelles and cytoplasm was accompanied by an increase in chromatin compaction.
Water stress increase is conducive to DNA damage and results in germination decrease, as in Avicennia marina is observed by Boubriak et al. (2000). Crévecouer et al. (1976) observe that, during the germination of corn, chromatin compaction in the embryonic tissues accompanies dehydration damage. In E. edulis embryo tissues, chromatin damage was also visible during desiccation. In fact, increased chromatin compaction was one feature of the initially reversible deterioration of the E. edulis seeds, and this feature preceded the critical water content of germination. Boubriak et al. (1997) propose that the capacity of Secale cereale and Avena fatua dehydrated embryos to repair DNA when they are re-hydrated is a critical event in the maintenance of a functional genome and is an essential component in the drying tolerance mechanism. Similarly, Boubriak et al. (2000) show the loss of the capacity for DNA repair when 22% of the total water is lost from highly recalcitrant Avicennia marina root primordium. After a critical degree of tissue dehydration, the capacity for DNA repair in E. edulis embryos may also be lost and, thus, may be a possible cause for the abrupt decrease in germination.
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (Argentina), BID 1201/OC-AR PICT 08-04536.
We thank Justo Herrera and Karina Schiaffino, Centro de Investigaciones Ecológicas Subtropicales (CIES), Parque Nacional Iguazú, Argentina for field assistance and their help in obtaining specimens. S. Maldonado is a career member of CONICET. V. Láinez is a professional technician of CONICET.
References
1. Andrade ACS (2001). The effect of moisture content and temperature on the longevity of heart of palm seeds (Euterpe edulis). Seed Sci Tech 29: 171-182. [ Links ]
2. Beewar M, Stanwood R, Roos ER (1982). Dehydration effects on imbitional leakage from a sensitive seeds. Plant Physiol 69: 1132-1135. [ Links ]
3. Belin-Depoux M, Hering de Queiroz M (1971). Contribution à l' étude ontegénique del palmiers. Quelques aspects de la germination de Euterpe edulis Mart. Rev Gen Bot 78: 339-371. [ Links ]
4. Berjak P, Farrant JM, Pammenter NW (1989). The basis of recalcitrant seed behaviour, In: Recent advances in the development and germination of seeds. R. V. Taylorson, Plenum Press, New York, pp 89-108. [ Links ]
5. Bonner FT (1996). Response to drying of recalcitrant seeds of Quercus nigra L. Ann Bot 78: 181-187. [ Links ]
6. Boubriak I, Kargiolaki H, Lyne L, Osborne DJ (1997). The requirement for DNA repair in desiccation tolerance of germinating embryos. Seed Sci Res 7: 97-105. [ Links ]
7. Boubriak I, Dini M, Berjak P, Osborne DJ. (2000). Desiccation and survival in the recalcitrant seeds of Avicennia marina: DNA replication, DNA repair and protein synthesis. Seed Sci Res 10: 307-315. [ Links ]
8. Crévecouer M, Deltour R, Bronchart R. (1976). Cytological study on water stress during germination of Zea Mays. Planta 132: 31-41. [ Links ]
9. Farrant JM, Berjak P, Pammenter NW. (1985). The effect of drying rate on viability retention of recalcitrant propagules of Avicennia marina. S Afr J Bot 51: 432-438. [ Links ]
10. Finch Savage WE (1992). Embryo water status and survival in the recalcitrant species Quercus robur L: evidence for critical moisture content. J Exp Bot 43: 663-669. [ Links ]
11. ISTA (1989). Report ISTA Committees, The 25th ISTA Congress 1998. Seed Sci. Tech 26, Supplement 1. [ Links ]
12. ISTA (2005). International rules for seed testing. Edition 2005. The International Seed Testing Association (ISTA) Bassersdorf. [ Links ]
13. Kundu M, Kachari J. (2000). Desiccation sensitivity and recalcitrant behavior of seeds of Aquilaria agallocha Roxb. Seed Sci Tech 28: 755-760. [ Links ]
14. Maldonado S, Lott JNA (1991). Protein bodies in Datura stramonium seeds: structure and mineral nutrient composition. Can J Bot 69: 2545-2554. [ Links ]
15. Martins CC, Nakagawa J, Alves Bovi ML (2000). Desiccation tolerance of four seed lots from Euterpe edulis Mart. Seed Sci Tech 28: 101-113. [ Links ]
16. Pammenter NW, Berjak P (1999). A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Sci Res 9: 13-37. [ Links ]
17. Panza V, Láinez V, Prego I, Maroder H, Maldonado S (2001). Efecto del secado sobre parámetros germinativos en Euterpe edulis Mart. (Effects of desiccations on Euterpe edulis germinative parameters) p. in actas XII Congresso Brasileiro de Sementes. Curitiba, Brasil. [ Links ]
18. Panza V, Láinez V, Maldonado S (2004). Structural and chemical studies on Euterpe edulis seed. Bot J Linn Soc 145: 445-453. [ Links ]
19. Pritchard HW (1991). Water potential and embryonic axis viability in recalcitrant seeds of Quercus rubra. Ann Bot 67: 43-49. [ Links ]
20. Pritchard HW, Haye AJ, Wright WJ, Steadman KJ (1995). A comparative study of seed viability in Inga species: desiccation tolerance in relation to the physical characteristics and chemical composition of the embryo. Seed Sci Tech 23: 85-100. [ Links ]
21. Queiroz MH de, Cavalcante MDT de H (1986). Efeito do dessecamento das sementes de palmiteiro na germinação e no armazenamento. (Effects of desiccations on Euterpe edulis germination and storage). Rev Br Sem 8: 121-125. [ Links ]
22. Reis A, Silveira Paulilo MT, Nakazono EM, Venturi S (1999). Effect of different level of desiccation in the seed germination of Euterpe edulis Martius - Arecaceae. INSULA 28: 31-42. [ Links ]
Received on November 17, 2006. Accepted on August 14, 2007.














