Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.32 n.1 Mendoza jan./abr. 2008
Spherites in the midgut epithelial cells of the sugarcane borer parasitized by Cotesia flavipes
Daniela de Oliveira Pinheiro*, Hélio Conte**, and Elisa Aparecida Gregório*
* Departamento de Morfologia, Instituto de Biociências, Universidade Estadual Paulista-UNESP, Rubião Júnior, s/n, CEP: 18618-000, Botucatu, SP, Brazil.
** Departamento de Biologia Celular e Genética, Universidade Estadual de Maringá-UEM, Av. Colombo 5790, CEP 87020-900, Maringá- PR, Brazil.
Address correspondence to: Dra. Daniela de Oliveira Pinheiro. Centro de Microscopia Eletrônica, IB, UNESP, Campus de Botucatu, Botucatu, SP. CEP 18618-000, BRAZIL. E-mail: daniela_pinheiro@yahoo.com
ABSTRACT: Diatraea saccharalis, the main pest of sugarcane, has been controlled by Cotesia flavipes. Very little is known about the effect of parasitism on the host organs, including the midgut. The Lepidoptera midgut epithelium is composed of columnar, goblet, regenerative, and endocrine cells. Spherites have been described in columnar and regenerative cells of several Lepidoptera species, and presented a lot of functional meaning. We identified spherites in the midgut epithelial cells of non-parasitized D. saccharalis larvae analyzed the effect of parasitism on spherite morphology and distribution along the length of the midgut. Midgut fragments of both non-parasitized and parasitized larvae were processed for transmission electron microscopy. All the midgut epithelial cells showed spherites, but they were not preferentially located in a particular part of the cells. Parasitized larvae had more spherites, mainly in the columnar cells, than non-parasitized larvae. This observation was associated with an ionic imbalance within the insect host. Spherites were more abundant in the anterior midgut region than in other regions, which suggests that this region is involved in ion transport by intracellular and/or paracellular route. The morphological variability of spherites in the cells of parasitized larvae was related to the developmental stages of these structures.
Keywords: Spherite; Epithelial cell; Ultrastructure; Lepidoptera; Parasitism
Introduction
Spherites are spherical cytoplasmatic granules whose mineral content assume the form of concentric lamination. These structures have been described in cells of different organs in invertebrates. In addition to the presence of spherites in the insect midgut (Wright and Newel, 1964; Cruz-Landim, 1971, 2000; Nopanitaya and Misch, 1974; Turbeck, 1974; Waku and Sumimoto, 1974; Sohal et al., 1977; Humbert, 1978; Serrão and Cruz-Landim, 1996, 2000; Cruz-Landim and Serrão, 1997), these structures are also present in insect Malpighian tubules (Teigler and Arnott, 1972; Ryerse, 1979; Krüger et al., 1987; Hazelton et al., 1988; Spring and Felgenhauer, 1996; Cruz-Landim, 2000; Hazelton et al., 2001), in the midgut glands of invertebrates (Ludwig and Alberti, 1988; Lipovsek et al., 2002, 2004; Pigino et al., 2006), and in the mantle tissue of bivalves (Vesk and Byrne, 1999).
The chemical composition of these structures varies in invertebrates. Turbeck (1974) verified that spherites represent a calcium reserve, which also possesses magnesium in their structure, for several Lepidoptera species. Lipovsek et al. (2002) demonstrated that young spherites of bivalves are composed of calcium and phosphorus in electron-dense layers and silicon in electron-lucent layers. The authors show that the layers of spherites contain organic material, such as glycoprotein and proteoglycans, associated with the inorganic compounds cited.
Little is known about the origin of these structures in the cells of insects. For Hymenoptera, it was proposed that the organic material, a precursor of spherites, may be produced within the rough endoplasmic reticulum, and the inorganic material also may be sequestered by this organelle, which may lose ribosomes that create the membrane surrounding the crystalloid (Cruz-Landim, 2000).
The sugarcane borer, Diatraea saccharalis Fabricius, is the main sugarcane pest and also affects other crops such as sorghum, corn, and rice. This pestilential insect has been controlled by the release, in nature, of the parasitoid Cotesia flavipes (Hymenoptera: Braconidae). The midgut epithelium in Lepidoptera is principally made up of columnar, goblet, regenerative, and endocrine cells (Lehane and Billingsley, 1996). Many studies suggest that the distribution, morphology, and morphometry of these cells may be variable throughout the length of the midgut (Santos et al., 1984; Pinheiro and Gregório, 2003; Pinheiro et al., 2003). We studied the ultrastructure of the spherites in midgut epithelial cells of D. saccharalis larvae, both non-parasitized and parasitized by C. flavipes. We correlated these findings with the spatial distribution of cells along the midgut.
Material and Methods
The insects were reared on an artificial diet and maintained under controlled conditions (26 ± 1ºC; 70± 5% humidity, and 14 h photophase). Cotesia flavipes females were left to oviposit on the dorsal surface of the last instar Diatraea saccharalis larvae. Parasitized and non-parasitized larvae were sampled at six days after parasitism. The insects were dissected; the midgut was removed and fragmented in the anterior and posterior regions. The midgut fragments were fixed in 2.5% glutaraldehyde - 4% paraformaldehyde solution in 0.1 M phosphate buffer (pH 7.3) for 24 h, post-fixed in 1% osmium tetroxide in the same buffer for 2 h, dehydrated through a graded series of acetone, and embedded in Araldite7 resin. Ultrathin sections were double-stained with uranyl acetate and lead citrate and examined under a Philips CM100 transmission electron microscope.
Results
In non-parasitized D. saccharalis larvae, spherites were scarce in the cytoplasm of all epithelial cell types, in both the anterior and in the posterior midgut regions (Figs. 1-6). The spherites were not preferentially located within a particular part of the cell, and they varied in form, size, and structure. The spherites were electrondense lamellas, which were located in the vacuolar membrane enwrapping, or which were filling the vacuole with several lamellas of variable thickness and concentric disposition (Figs. 1-6).
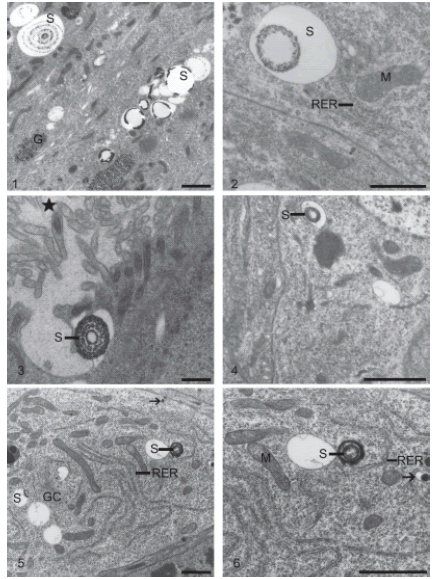
FIGURES 1-6. Epithelial cells from the midgut of non-parasitized Diatraea saccharalis larvae: spherites (S); glycogen (G); rough endoplasmic reticulum (RER); goblet cell cavity («); granules (arrow); mitochondria (M); Golgi complex (GC). Fig. 1. Columnar cell. Bar = 1μm. Fig. 2. Columnar cell. Bar = 0.5μm. Fig. 3. Goblet cell. Bar = 1μm. Fig. 4. Regenerative cell. Bar = 1μm. Fig. 5. Endocrine cell. Bar = 1μm. Fig. 6. Endocrine cell. Bar = 1μm.
In parasitized larvae, the spherites were abundant and varied in size and form; they were present in the cytoplasm of all epithelial cell types, most frequently columnar cells, especially those of the anterior midgut region (Figs. 7-11). The spherites were voluminous and generally occurred in thinner lamellas than those observed in non-parasitized larvae (Figs. 7-13). The internal morphology was also variable. Some spherites did not occur in structured lamellas, and they contained finely flocculated material of low electron density that were concentrated close to the vacuolar membrane in some cases (Figs. 7, 9-10). Other spherites possessed only a thin lamella or innumerable concentric lamellas (Figs. 7-9, 11-13). The grouped or isolated spherites were not preferentially located within a particular part of the epithelial cells (Figs. 7-13). The spherite membranes were frequently fused (Figs. 8-11). But scarce spherites were noted in the posterior midgut region, and those observed were small and with few lamellas (Figs. 12-13). We did not observe the release of spherites into the midgut lumen in any epithelial cell from the two midgut regions.
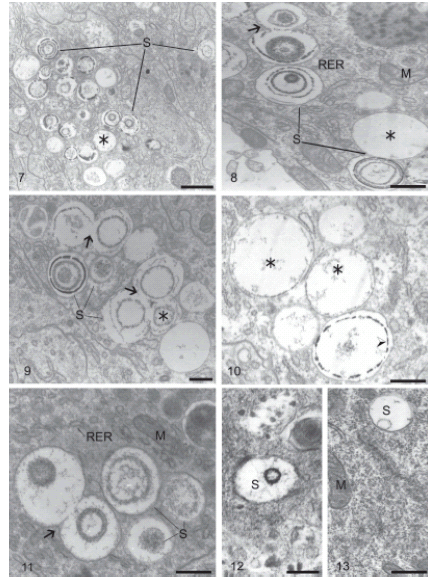
FIGURES 7-13. Columnar cells of the anterior (Figs. 7-11) and the posterior regions (Figs. 12-13) of the midgut of Diatraea saccharalis larvae parasitized by Cotesia flavipes: spherite (S); rough endoplasmic reticulum (RER); mitochondria (M); fusion of spherite membranes (arrow); flocculated material (¬). Fig. 7. Spherites of heterogeneous morphology. Bar = 1μm. Fig. 8. Spherites of heterogeneous morphology. Bar = 0.5μm. Fig. 9. Innumerable spherites with electron-lucent lamellas among flocculated material, and few spherites with electron-dense lamellas. Bar = 0.5μm. Fig. 10. Spherites with varied morphology; concentration of electron-dense material (arrowhead) near vacuolar membrane of spherite. Bar = 0.5μm. Fig. 11. Spherites with thick structured lamellas, a few being electrondense. Bar = 0.5μm. Fig. 12. Spherite with electron-dense lamella. Bar = 0.25μm. Fig. 13. Spherite with electron-lucent lamella. Bar = 0.5μm.
Discussion
To date, spherites have been described only in columnar and regenerative cells of the insect midgut (Turbeck, 1974; Cruz-Landim, 1971). Therefore, an interesting finding of our study is the spheres, crystals, or spherites inside the cytoplasm of the four cell types in the midgut epithelium of D. saccharalis, both non-parasitized and parasitized by C. flavipes.
Independent of the type and location of midgut epithelial cells in D. saccharalis, the spherite morphology varies as much in relation to crystalloid inclusion ultrastructure as in relation to the size and form of the surrounding vacuole. Spherites have been described, by use of electron transmission microscopy, as membranous vesicles containing layers of electron-dense material, interspersed with other electron-lucent content, which form concentric lamellas (Cruz-Landim, 1971, 2000; Nopanitaya and Misch, 1974; Turbeck, 1974; Serrão and Cruz-Landim, 1996; Corrêa et al., 2002, 2003; Lipovsek et al., 2002, 2004). In a study by Wright and Newell (1964), columnar cells from Anystis sp did not show with precision the spherite vacuolar membrane. This finding was probably the result of technical problems, considering that subsequent studies, after improvement in fixation techniques, clearly showed that the spherites are delimited by membrane (Nopanitaya and Misch, 1974; Turbeck, 1974; Serrão and Cruz-Landim, 1996; Cruz-Landim, 2000; Corrêa et al., 2003).
The location of spherites in insect epithelial cells has been described as varied. Turbeck (1974) showed, in seven Lepidoptera species, that spherites were preferentially located in the apical cytoplasm in both columnar and regenerative cells. The same was described by Cruz-Landim (1971) for midgut columnar cells of Trigona postica. However, Serrão and Cruz-Landim (2000), in studying the midgut of Meliponinae larvae, observed spherites in the middle portion of columnar cells. In the midgut of D. saccharalis, in both non-parasitized and parasitized larvae, spherites did not show a preferential location within a particular part of the cell.
In midgut epithelial cells of parasitized D. saccharalis larvae, we observed more spherites in epithelial cells, especially in columnar cells of the anterior midgut region than in other cell types. The abundance of spherites in the anterior midgut region in Diptera, in a normal situation of insect development, was reported by Nopanitaya and Mish (1974). Cruz-Landim (1971), in studying midgut columnar cells of T. postica, related that the accumulation of spherites at the end of larval development causes cellular hypertrophy. A morphometric study of epithelial cells in D. saccharalis parasitized by C. flavipes showed that parasitism induces cellular increase only in columnar cells of the posterior midgut region and that this process is independent of the increase in abundance of spherites (Pinheiro et al., 2006); thus, we believe that the spherite abundance, in our observations, principally in anterior columnar cells, cannot be related to cellular hypertrophy.
The function of spherites in epithelial cells of the midgut or other insect organs is not yet totally understood. One of the functions postulated is related to cellular degeneration. For example, Turbeck (1974) observed that spherites in midgut epithelial cells appear in larvae before ecdysis and disappear during cellular differentiation. The presence of spherites close to the Golgi complex in midgut secretory cells of Anystis sp led Waku and Sumimoto (1974) to propose the participation of this structure in the secretion process. Other authors have reported that this structure is associated with cellular excretion of ions and detoxification (Wright and Newel, 1964; Cruz-Landim, 1971, 2000; Ludwig and Alberti, 1988; Serrão and Cruz-Landim, 1996; Lipovsek et al., 2002, 2004). However, according to Sohal et al. (1977), the presence of spherites may be associated with food ingestion or insect age; the richer in metals the food, or the older the larvae, the greater the spherite quantity observed in the columnar cell cytoplasm.
As the spherites containing calcium disappear after pupation, some authors associate the structures with mineralization of the cuticle (Ludwig and Alberti, 1988; Lipovsek et al., 2002). We cannot confirm or discard this association, because our observations in D. saccharalis are restricted to the beginning of the fifth instar. As the parasitized and non-parasitized larvae had access to the same diet, we discarded the possibility that the greater number of spherite in the parasitized larvae was related to a variation in the diet of the insect.
Some authors have observed the release of spherites in to the intestinal lumen (Wright and Newel, 1964; Gouraton, 1968 apud Serrão and Cruz-Landim, 1996; Cruz-Landim, 1971). The same phenomenon was observed in Malpighian tubules of some insects (Hazelton et al., 1988; Spring and Felgenhauer, 1996). It is known that Malpighian tubules are responsible for the maintenance of the hydric and ionic equilibrium of the insect (Chapman, 1998). The spherites are related to the process of rapid transport of fluids and to excretion of the heavy metals, organic materials, and inorganic materials stored by spherites (Hazelton et al., 2001). It is also known that midgut columnar cells aid goblet cells in ionic homeostasis and metabolite absorption by intracellular routes (Lehane and Billingsley, 1996; Terra et al., 2006). Recently, Fiandra et al. (2006) observed that the transport of electrolytes, including calcium ions, by paracellular routes also occurs in Lepidoptera midgut columnar cells equivalent to that proposed for Malpighian tubules (Beyenbach et al., 2000), exercising intracellular modulation mechanisms of this process in the midgut.
We believe that the parasitism of D. saccharalis by C. flavipes may be provoking ionic disequilibrium, which induces an increases in the number of spherites in the columnar cells; furthermore, the presence of innumerable spherites in anterior midgut columnar cells point to the importance of these structures in ion transport by an intracellular and/or paracellular route in this midgut region, a subject which requires more research. Consistent with our findings, it was recently verified that midgut spherites represent an ionic barrier regulating the quantity of metal ions that enter the organism of invertebrates through the alimentary tract (Pigino et al., 2006).
In Malpighian tubule cells of Acheta domesticus, direct interaction was observed between spherites and mitochondria (Hazelton et al., 2001); the authors postulate that inorganic phosphate and calcium ions must be passing between the outer membrane of mitochondria and the membrane surrounding the spherites and, therefore, rendering this intimate association favorable for both organelles. In D. saccharalis we did not observe an association between spherites and mitochondria, which suggests that the ionic movement must be occurring by other mechanisms.
Although the fusion between spherite membranes has been frequently observed in midgut columnar cells of parasitized insects, the reason for this fusion is still not known. In columnar cells of bees, the fusion between spherites and autophagic vacuoles, eliminated together to the intestinal lumen, has been observed (Serrão and Cruz-Landim, 1996; Lipovsek et al., 2002).
In parasitized larvae, the numerous spherites observed showed wide variability in the morphology of its content. The majority of the spherites had low densities, contained sparse flocculated material concentrated on the periphery of the vacuolar membrane, and formed a discrete electron-dense layer or a few concentric lamellas. However, in non-parasitized larvae, the few spherites observed contained thick and contrasting lamellas. We believe that the less structured spherites may be younger spherites that were formed as a result of metabolic alterations, especially in the ionic equilibrium, of the host insect during the six-day period of parasitism. The morphological variability of these spherites, especially in parasitized larvae, may also be attributed to instability in the content of these structures as a consequence of ionic alterations related to metabolism.
Acknowledgments
We are grateful to the Entomological Laboratory of Usina Barra Grande, Lençóis Paulista-SP, Brazil, for rearing the insects and to Ms. M. H. Moreno, Ms. C. S. Tardivo, Mrs. N. A. Basso and Mrs. V. A. Salvador for their excellent technical assistance. The authors are indebted to Dra. Edy de Lello Montenegro and Dr. Luis Antonio Toledo for their scientific collaborations. This work was partially supported by FAPESP.
References
1. Beyenbach KW, Pannabecker TL, Nagel W (2000). Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J Exp Biol. 203: 1459-1468. [ Links ]
2. Chapman RF (1998). The Insects: structure and function. 4aed. Harvard University Press, Cambridge, 770p. [ Links ]
3. Corrêa Jr JD, Allodi S, Farina M (2003). Enzymatic, analytical and structural aspects of electron-dense granules in cells of Ucides cordatus (Crustacea, Decapoda) hepatopancreas. Cell Tissue Res. 311: 107-116. [ Links ]
4. Corrêa Jr JD, Silva MR, Silva ACB, Lima SMA, Malm O, Allodi S (2005). Tissue distribution, subcellular localization and endocrine disruption patterns induced by Cr and Mn in the crab Ucides cordatus. Aquatic Toxicol. 73: 139-154. [ Links ]
5. Corrêa Jr JD, Farina M, Allodi S (2002). Cytoarchitectural features of Ucides cordatus (Crustacea, Decapoda) hepatopancreas: structure and elemental composition of electron-dense granules. Tissue Cell. 34: 315-325. [ Links ]
6. Cruz-Landim C (2000). Localization of calcium and acid phosphatase in the Malpighian tubules of nurse workers of Melipona quadrifasciata anthidioides Lep. (Hymenoptera, Apidae, Meliponini). Biosci J. 16: 87-99. [ Links ]
7. Cruz-Landim C (1971). Note on granules with concentric lamination present in the larval midgut of Trigona (Scaptotrigona) postica Latr. (Hym. Apidae). Rev Bras Pesqui Méd Biol. 4: 13-16. [ Links ]
8. Cruz-Landim C, Serrão JE (1997). Ultrastructural biochemistry of the mineral concretions in the midgut of bees (Hymenoptera: Apidae). Neth J Zool. 47: 21-29. [ Links ]
9. Fiandra L, Casartelli M, Giordana B (2006). The paracellular pathway in the lepidopteran larval midgut: Modulation by intracellular mediators. Comp Biochem Physiol A. Mol Integr Physiol. 144: 464-473. [ Links ]
10. Hazelton SR, Felgenhauer BE, Spring JH (2001). Ultrastructural changes in the Malpighian tubules of house cricket, Acheta domesticus, at the onset of diuresis: a time study. J Morphol. 247: 80-92. [ Links ]
11. Hazelton SR, Parker SW, Spring JH (1988). Excretion in the house cricket, (Acheta domesticus) fine structure of the Malpighian tubules. Tissue Cell. 20: 443-460. [ Links ]
12. Humbert W (1978). Cytochemistry and x-ray microprobe analysis of the midgut of Tomocerus minor Lubbock (Insecta, Collembola) with special reference to physiological significance of the mineral concretions. Cell Tissue Res. 187: 397-416. [ Links ]
13. Krüger RA, Broce AB, Hopkins TL (1987). Dissolution of granules in the Malpighian tubules of Musca autumnalis Degeer, during mineralization of puparium. J Insect Physiol. 33: 255-263. [ Links ]
14. Lehane MJ, Billingsley PF (1996). Biology of the insect midgut. Chapman and Hall, London, 486p. [ Links ]
15. Lipovsek S, Letofsy-Papst I, Hofer F, Pabst MA (2002). Seasonal and age-dependent changes of the structure and chemical composition of the spherites in the midgut gland of the harvestmen Gyas annulatus (Opiliones). Micron. 33: 647-654. [ Links ]
16. Lipovsek S, Novak T, Janzekovic F, Sencic L, Pabst MA (2004). A contribution to the functional morphology of midgut gland in phalangiid harvestmen Gyas annulatus and Gyas titanus during their life cycle. Tissue Cell. 36: 275-282. [ Links ]
17. Ludwig M, Alberti G (1988). Mineral congregations, spherites in the midgut gland of Coelotes terrestris (Aranae): structure, composition and function. Protoplasma. 143: 43-50. [ Links ]
18. Nopanitaya W, Misch DW (1974). Developmental cytology of the midgut in the Flesh-fly, Sarcophaga bullata (Parker). Tissue Cell. 6: 487-502. [ Links ]
19. Pigino G, Migliorini M, Paccagnini E, Bernini F (2006). Localization of heavy metals in the midgut epithelial cells of Xenillus tegeocranus (Hermann, 1804) (Acari: Oribatida). Ecotoxic Envirom Safety. 64: 257-263. [ Links ]
20. Pinheiro DO, Silva RJ, Gregório EA (2006). Morphometry of the Diatraea saccharalis Fabricius, 1794 (Lepidoptera) midgut epithelium parasitized by the wasp Cotesia flavipes Cameron, 1891 (Hymenoptera). J Invertebr Pathol. 93: 60-62. [ Links ]
21. Pinheiro DO, Silva RJ, Quagio-Grassiotto I, Gregório EA (2003). Morphometric study of the midgut epithelium in the Diatraea saccharalis Fabricius (Lepidoptera: Pyralidae) larvae. Neotrop Entomol. 32: 453-459. [ Links ]
22. Pinheiro DO, Gregório EA (2003). Ultrastructure of the columnar epithelial cell along the midgut of the Diatraea saccharalis (Lepidoptera: Pyralidae) larvae. Acta Microsc. 12: 27-30. [ Links ]
23. Ryerse JS (1979). Developmental changes in Malpighian tubules cell structure. Tissue Cell. 11: 533-551. [ Links ]
24. Santos CD, Ribeiro AF, Ferreira C, Terra WR (1984). The larval midgut of the cassava hornworm (Erinnyis ello): ultrastructure, fluid and fluxes and the secretory activity in relation to the organization of digestion. Cell Tissue Res. 237: 565-574. [ Links ]
25. Serrão JE, Cruz-Landim C (1996). Ultrastructure of digestive cells in stingless bees of various ages (Hymenoptera, Apidae, Meliponinae). Cytobios. 88: 161-171. [ Links ]
26. Serrão JE, Cruz-Landim C (2000). Ultrastructure of the midgut epithelium of melyponinae larvae with different developmental stages and diets. J Apicultural Res. 39: 9-17. [ Links ]
27. Sohal RS, Peters PD, Hall TA (1977). Origin, ultrastructure, composition and age dependence of mineralized dense bodies (concretions) in the midgut epithelium of the adult housefly, Musca domestica. Tissue Cell. 9: 87-102. [ Links ]
28. Spring JH, Felgenhauer BE (1996). Excretion in the house cricket, Acheta domesticus: effect of diuretics on the structure of the mid-tubule. J Morphol. 230: 43-53. [ Links ]
29. Terra WR, Costa RH, Ferreira C (2006). Plasme membranes from insect midgut cells. Ann Braz Acad Sci. 78: 255-269. [ Links ]
30. Teigler DJ, Arnott HJ (1972). Crystal development in the Malpighian tubules of Bombyx mori (L.). Tissue Cell. 4: 173-185. [ Links ]
31. Turbeck B (1974). A study of the concentrically laminated concretions spherites in the regenerative cells of the midgut of Lepidopterous larvae. Tissue Cell. 6: 627-640. [ Links ]
32. Vesk PA, Byrne M (1999). Metal levels in tissue granules of the freshwater bivalve Hyridella depressa (Unionida) for biomonitoring: the importance of cryopreparation. Sci Total Environ. 225: 219-229. [ Links ]
33. Waku Y, Sumimoto KI (1974). Metamorphosis of midgut epithelial cells in the silkworm (Bombyx mori L.) with special regard to the calcium salt deposits in the cytoplasm. II. Electron microscopy. Tissue Cell. 6: 127-136. [ Links ]
34. Wright KA, Newel midgut (1964). Some observation on fine structure of the midgut of the mite Anystis sp. Ann Entomol Soc Am. 57: 684-693. [ Links ]
Received on November 20, 2006.
Accepted on September, 2007.














