Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.32 no.2 Mendoza June/Aug. 2008
Cell proliferation of the ileum intestinal mucosa of diabetic rats treated with ascorbic acid
Jacqueline Nelisis Zanoni and Renata Virginia Fernandes Pereira
Departament of Morphophysiological Sciences, State University of Maringá, Maringá/PR, Brasil.
Address correspondence to: Dra. Jacqueline Nelisis Zanoni. Departamento de Ciencias Morfofisiol—gicas, Universidade Estadual de Maring‡. Av. Colombo, 5790 Bloco H-79 - CEP 87020-900. Maringá/PR, BRASIL. FAX: (+55-44) 3261 4340. E-mail: jnzanoni@uem.br
ABSTRACT: The objective of this work was to evaluate the effect of the ascorbic acid supplementation on the cellular proliferation on the ileum mucosa of diabetic rats. Fifteen 90-days rats were divided in the groups: control, diabetic and diabetic supplemented with ascorbic acid (DA). Two hours prior the sacrifice, they were injected with Vincristin. Semi-seriate histological cuts stained with HE were accomplished. About 2500 crypt cells from the intestinal mucosa were counted in order to obtain the metaphasic indexes. The height and depth of 30 villi and 30 crypts were measured for each animal, respectively. The metaphasic indexes showed no significant changes when we compared the three groups: 20.2 ± 0.7 (control), 18 ± 1.9 (diabetic) and 17 ± 1.4 (DA) (p > 0.05). The values obtained from the crypts measurement were 221.2 ± 8.5 (control), 225.3 ± 9.5 (diabetic) and 222 ± 34 (DA). The villi of the control, diabetic and DA animals presented the following results: 301.7 ± 25.33, 304.8 ± 25.63 and 322.1 ± 45.77 respectively. The morphometric data were not different statistically (p > 0.05). Summing up, the present work showed that there was no alteration in the cellular proliferation of the ileum of diabetic-induced rats supplemented with ascorbic acid.
Key words: Ascorbic acid; Diabetes mellitus; Lleum; Cellular proliferation.
Introduction
The diabetes mellitus is a chronic disease characterized by hyperglycemia, with alterations in the cellular metabolism. The complications arising from the disease are the main responsible for the damages caused to the diabetic patient, such as dysfunction and failure of several organs, retinopathy, neuropathy and outlying vasculopathy (Zhao et al., 2003).
The gastrointestinal tract is one of the most intensely attacked areas during the diabetes mellitus development. Due to the diabetes mellitus the small intestine goes through morphologic alterations in the mucosa (Lorenz-Meyer et al., 1977; Zoubi et al., 1995a), compromising the movement of food through the intestinal lumen, the secretion of enteric juices and the absorption of digestion products, which depend on the integrity of the epithelium covering this organ.
The diabetic hyperphagia is one of the responsible for the hyperplasia of the intestinal epithelium, although its action mechanism is still ignored (Miller et al., 1977; Zoubi et al., 1995b; Fischer et al., 1997; Noda et al., 2001; Zhao et al., 2003). It is believed that the interference of the food ingested at the apoptosis, which plays an important role in the physiologic or pathological death of the epithelial cells maintaining the homeostasis of the mucosa, it can be involved in that process (Noda et al., 2001).The intestinal innervation, although less studied, also plays an important role in the cellular kinetics regulation (Tutton, 1975; Klein and Torres, 1978; Klein, 1979; Klein and McKenzie, 1980; Zucoloto et al., 1988; Holle et al., 1989; See et al., 1990; Holle, 1991; Zucoloto et al., 1991; Hadzijahic et al., 1993; Zucoloto et al., 1997). The cellular proliferation, the migration and the apoptosis depend on a circadian rhythm that is abolished with the denervation (Silva, 2004). The diabetes mellitus causes a decrease of the enteric innervation with the consequent occurrence of the diabetic neuropathy, intimately related to the changes of the gastrointestinal tract.
The neuronal damage due to the diabetes mellitus is attributed mainly to the sorbitol, a substance produced by the glucose reduction in the reaction catalyzed by the enzyme aldose redutase (Vinson et al., 1989). Therefore, the sorbitol is responsible for the edema occurrence, neuronal lesion and a consequent reduction on the nervous conduction speed (Hosking et al., 1978). The sorbitol concentration can be reduced in the diabetes through the use of the ascorbic acid, an inhibitor of the enzyme aldose redutase (Yue et al., 1989; Cunningham et al., 1994; Will and Byers, 1996; Cunningham, 1998a). However, vitamin C is not a substance synthesized by human beings which can have their tissual concentrations reduced in the diabetes mellitus, since its transport is inhibited during the hyperglycemia, as well as its renal reabsorption (Cunningham, 1998b).
The myenteric neurons death in the diabetes can also be attributed to the disequilibrium in the free radicals production, which is responsible for cumulative and irreversible cellular damages, such as loss of cellular functions and even death for necrosis or apoptosis (Kuyvenhoven and Meinders, 1999; Imai and Nakagawa, 2003). Besides, the occurrence of the oxidative stress is also responsible for the decrease of the antioxidant substances as the ascorbic acid (Young et al., 1992), which prevents the formation of free radicals or neutralizes the species already formed.
Therefore, the use of ascorbic acid can be beneficial in the maintenance of the intestinal epithelium integrity in the diabetes mellitus. This substance can function as an antioxidant and decrease the sorbitol concentration, having a neuroprotector role on the enteric nervous system neurons and promoting a neural regulation of the intestinal mucosa proliferation. The objective of this work was to study the effect of the supplementation with the ascorbic acid on the ileum mucosa of diabetic rats.
Material and Methods
Obtaining the study groups
All the experiments described in this manuscript were reviewed and approved by the State University of Maringá Animal Uses Committee. Fifteen male albino Wistar rats (Rattus norvegicus) were used to perform this study. The animals, aged ninety days (body weight 300-350 g), were kept in individual metabolic cages in a room with a maintained photoperiod (6:00 a.m. - 6:00 p.m.) and room temperature (RT) (24ºC ± 2ºC). Water was given ad libitum and Nuvital® lab chow served as the diet.
The fifteen 90-days rats were divided in three groups: control, diabetic and diabetic supplemented with ascorbic acid (DA). The diabetic and DA animals received a single Streptozotocin (Sigma, St. Louis, MO, USA) injection, with a dose of 35 mg·kg-1 body weight, freshly dissolved in 10 mmol·l-1 citrate buffer (pH 4,5). The animals were submitted to a previous fourteen-hours fast before receiving the Streptozotocin. Successful injection of diabetes was confirmed by the presence of glycosuria and weight loss.
The animals from DA group were supplemented with ascorbic acid (1 g·l-1 day) (Sigma, St. Louis, MO, USA) for 120 days (Young et al., 1992). The vitamin was added daily to the animals' water.
The animals' ileum were collected and processed for the elaboration of histological sections stained with hematoxylin-eosin (HE), to analyze the cellular proliferation through the determination of the metaphasic index and through the mucosa morphometric analysis.
Collecting and processing the material
The animals were sacrificed after four months of treatment, at the age of 210 days. Two hours prior the sacrifice, the animals were injected with 1 mg·kg-1 of body weight Vincristin (Oncovin, Eli Lilly, Brasil), a blocking agent of the mitotic spindle. The injections were always given at the same time to avoid circadian variations.
The rats were weighed and anesthetized with Thiopental (40 mg·kg-1) before being killed. The blood was collected by heart puncture in order to perform the count of glycemia (glucose oxidase method) (Bergmeyer and Bernet, 1974), glycated hemoglobin (Koenig et al., 1976) and ascorbic acid (Henry et al., 1988). At the end, the ileum was removed and carefully washed to remove feces. The segments were opened along the mesentericborder, fixed with pins into paper card with the mucosa surface upward. They were then immersed in buffered formalin for 6 hours.
After fixation, the ileum was dehydrated and embedded in 2-hydroxyethyl-metacrilate (Leica Historesine - Embedding Kit, Germany). Two micrometer semi-serial sections were made in a Leica RM 2145 microtome, with glass razor. The sectioned resins were stained by the HE.
Determining the metaphasic index
The metaphasic index was calculated by counting the interphasic and metaphasic epithelial nuclei in the ileum crypts. Approximately 2500 cells per animal were counted on BX 40 Olympus microscope under a 40 X lens. The metaphasic index was expressed as the percentage of nuclei in metaphase divided by the overall number of counted nuclei.
Morphometry of villus and crypts
The height of the villus and the depth of the crypts were measured in well-guided longitudinal sections. The crypts depth includes the extension between the junction crypt-villi and the crypt base. The villosities length includes the extension between the junction crypt-villi and the villosities ends. The morphometric assessment was carried out on 30 villus and 30 crypts per animal (150 measurements per group) through coupled ocular with Zeiss micrometer grade and 10X objective lens Olympus microscope (Hernandes et al., 2000).
Statistical treatment
Comparisons between groups were made using oneway ANOVA followed by the Tukey test for multiple comparisons. p < 0.05 was taken to indicate statistical significance. The results were expressed as mean (M)± standard error (SE). (n = number of rats).
Results
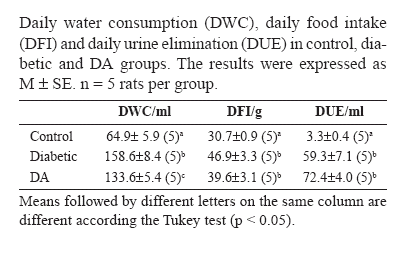
The onset of the diabetes mellitus in the diabetic and DA animals was confirmed as shown in the Tables 1 and 2. The analyses of water consumption, food intake and urine elimination demonstrated that the animals from both groups presented the characteristic clinical picture of the disease: polydipsia, polyphagia and polyuria (Table 1). The glycemia level showed that the diabetic rats and those from group DA were also severely hyperglycemic (Table 2).
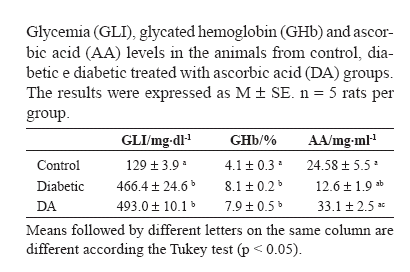
There were no differences in the glycated hemoglobin level between the diabetic and DA animals (p> 0.05) (Table 2). The plasmic ascorbic acid level, however, had a reduction in the diabetic animals when compared to the controls (p<0.05). The supplementation raised the levels of ascorbic acid in 25.7% in group DA when compared to the control (p > 0.05), and in 61.9% when compared to the diabetic (p < 0.05) (Table 2).
The group of diabetic animals and the group DA presented a reduction of 11% and 16% in their metaphasic index when compared to the control animals, respectively (p > 0.05). The index obtained for group DA was 5.6% smaller than the one observed for the diabetic (p > 0.05). The quantification data are shown in Figure 1.
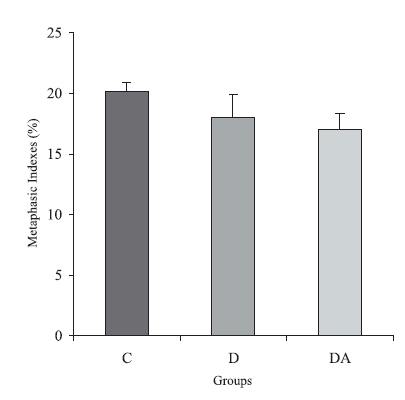
FIGURE 1. Metaphasic indexes from control, diabetic and diabetic treated with ascorbic acid (DA) groups. The results were expressed as M ± SE. n = 5 rats per group. p > 0.05 when all groups were compared.
The morphometric data obtained for the villi and intestinal crypts did not have significant changes after four months of diabetes and treatment with the ascorbic acid (p > 0.05). The group DA had villi 5.4% higher than the diabetic animals, and 6.3% larger than the controls (Fig. 2).
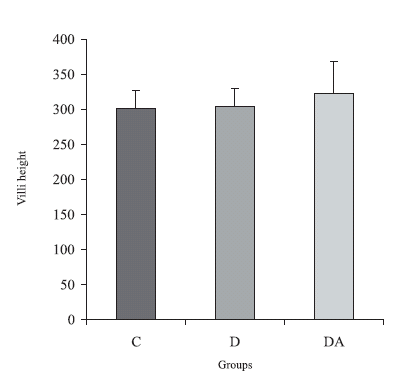
FIGURE 2. Villi height (mm) in the animals from control, diabetic and diabetic treated with ascorbic acid (DA) groups. The results were expressed as M ± SE. n = 5 rats per group. p > 0.05 when all groups were compared.
The crypts depth of group control was of 221.2 ± 8.5 µm, and 225.3 ± 9.5 µm for group diabetic. We did not find significant differences when comparing these two groups (p > 0.05). The crypts of group DA were 1.5% smaller in depth than the crypts evidenced in diabetic animals (p > 0.05).
Discussion
The integrity of the gastrointestinal tract lining epithelium depends on the balance between the proliferation and the cellular death (Zhao et al., 2003; Silva, 2004), which takes place in the primary anatomical and functional component, the unit crypt-villi. Having in mind the diabetes relationship with the proliferation and hypertrophy of cells in the intestinal mucosa, that proves an increase of the villi height and of the crypts depth (Miller et al., 1977; Zoubi et al., 1995b; Fischer et al., 1997; Noda et al., 2001; Zhao et al., 2003), we evaluated the influence of the chronic diabetes mellitus on the cellular proliferation of the ileum epithelium.
The diabetic condition was confirmed during the whole experiment in the diabetic and DA rats, through the polyuria, polydipsia and polyphagia and the severe hyperglycemia of these animals at the end of the experiment.
We did not observe alterations in the cellular proliferation of the diabetic animals and group DA after 120 days of the experimental induction of the diabetes mellitus. The metaphasic indexes obtained for these groups were similar to those observed in the control animals (p > 0.05). There are many factors that influence the cellular proliferation control in the epithelium of the gastrointestinal tract. The nutritional condition, the intestinal lumen environment, and some pathological conditions, such as the diabetes mellitus, interfere with the kinetics of the epithelial cells. Therefore, hormones with a trophic function in the intestine (as the growth hormone, glucagon, insulin and gastrin), vitamins, polyamines and peptides (as the glucagon-like 2 peptide - GLP-2) are involved with such control (Kedinger et al., 1987; Zhao et al., 2003; Silva, 2004).
The diabetes mellitus trophic effect on the cellular proliferation may also be related to the neuronal loss due to the disease, as demonstrated in the colon (Romano et al., 1996) and cecum (Zanoni et al., 1997). The cellular proliferation stimulus promoted by the chemical denervation with benzalkonium chloride (Zucoloto et al., 1988; Zucoloto et al., 1997; Hernandes et al., 2000) shows that the neuronal loss due to the diabetes mellitus, although not very much studied, may be a hypothesis for the alterations in the cellular division due to diabetes.
Despite the involvement of several factors already mentioned in the cell proliferation during the diabetes mellitus, we did not observe changes in the ileum epithelium in the present study. We believe that the period of time the rats were diabetic was decisive for the obtained results, since in all previously mentioned researches, the time the animals were diabetics was inferior to the one used in our experiment (four months). In studies such as Millet et al.´s (1977), the increase of the total cell number in the intestinal crypts of Simonsen rats was observed in the jejunum after 6 days of diabetes, with no differences found in the ileum. There is a possibility that the intestinal mucosa of the rats used in this research underwent an adaptation after a long period of diabetes.
Silva (2004) observed that the denervation influence on the mitotic index varies significantly depending on the kind of denervation, intestine area and, mainly, denervation time. The impact of the denervation by vagotomy and simpathectomy on the cell proliferation increased in the ileum during the acute phase, an effect that decreased gradually in the jejunum and in the ileum after 30 days, until it disappeared in the 90º day of denervation (Silva, 2004). Zucoloto et al. (1988) studied the cell proliferation in the duodenum intestinal mucosa of rats that had their myenteric plexus denervated with benzalkonium chloride during five months. They did not observe significant differences in the rate of the crypt cell production.
A study was performed on the enteric neurons of the ileum of diabetic rats supplemented with ascorbic acid in the same animals used in this experiment. It was observed that the diabetes mellitus caused a reduction in the number of the myenteric neurons overall population (immunoreactive to myosin-V), increase in the NADPH-d positive neurons area (Zanoni et al., 2003) as well as the submucous plexus VIP-ergic neurons (Zanoni et al., 2002). The supplementation with ascorbic acid presented a neuroprotector effect on these neurons. Although the supplementation with ascorbic acid has been positive on the enteric neurons, there were no changes in the intestinal mucosa.
The morphometric analysis accomplished in the present study also did not show alteration in the epithelial mucosa induced by the diabetes in the diabetic and DA animals after the experimental period of 120 days. The crypts depth and the villi height of animals from group diabetic were not statically different from the controls (p > 0.05). Different results were found when the period of diabetes was shorter than the time of experiment used in this work. The villi height and the crypts depth showed a significant increase after 4 days of diabetes in the duodenum and after 7 days of diabetes mellitus in the jejunum and ileum (p < 0.01) (Zhao et al., 2003). Miller et al. (1977) observed higher morphometric data of the intestinal crypts and villi after 6 days of diabetes mellitus (p < 0.05) however, those results were just found in the jejunum, not in the ileum.
Summing up, the present work showed that there was not alteration in the cellular proliferation of the ileum of experimentally induced diabetic rats and supplemented with ascorbic acid after a period of 120 days.
References
1. Bergmeyer HU, Bernet E (1974). Determination of glucose whit glucose-oxidase and peroxidase. In: Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York. [ Links ]
2. Cunningham JJ, Mearkle PL, Brown RG (1994). Vitamin C: an aldose reductase inhibitor that normalizes erythrocyte sorbitol in insulin-dependent diabetes mellitus. J Am Coll Nut. 13: 344-350. [ Links ]
3. Cunningham JJ (1998a). Micronutrients as nutriceutical interventions in diabetes mellitus. J Am Coll Nut. 17: 7-10. [ Links ]
4. Cunningham JJ (1998b). The glucose/insulin system and vitamin C: implications in insulin-dependent diabetes mellitus. J Am Coll Nut. 17: 105-108. [ Links ]
5. Fischer KD, Dhanvantari S, Drucker DJ, Brubaker PL (1997). Intestinal growth is associated with elevated levels of glucagon-like peptide 2 in diabetic rats. Am J Physiol. 273: E 815-820. [ Links ]
6. Hadzijahic N, Renehan WE, Ma CK, Zhang X, Fogel R (1993). Myenteric plexus destruction alters morphology of rat intestine. Gastroenterology 105: 1017-1028. [ Links ]
7. Henry RJ, Cannon DC, Winkelman JW (1988). Química clínica e bases técnicas. Editorial JIMS, Brazil. [ Links ]
8. Hernandes L, Zucoloto S, Alvares EP (2000). Effect of myenteric denervation on intestinal epithelium proliferation and migration of suckling and weanling rats. Cell prolif. 33: 127-138. [ Links ]
9. Holle GE, Granat T, Reiser SB, Holle F (1989). Effects of superior mesenteric and coeliac ganglionectomy on the small intestinal mucosa in the Hanford mini-pig. I. Histological and enzyme- histochemical study. J Auton Nerv Sys. 26: 135-145. [ Links ]
10. Holle GE (1991). Changes in the structure and regeneration mode of the rat small intestinal mucosa following benzalkonium chloride treatment. Gastroenterology. 101: 1264-1273. [ Links ]
11. Hosking DJ, Bennett T, Hampton JR (1978). Diabetic autonomic neurophaty. Diabetes 27: 1043-1055. [ Links ]
12. Imai H, Nakagawa Y (2003). Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 34: 145-169. [ Links ]
13. Kedinger M, Haffen K, Simon-Assmann P (1987). Intestinal tissue and cell cultures. Differentiation 36: 71-85. [ Links ]
14. Klein RM, Torres JJr (1978). Analysis of intestinal cell proliferation after guanethidine-induced sympathectomy. I. Stathmokinetic, labelling index, mitotic index, and cellular migration studies. Cell Tissue Res. 195: 239-250. [ Links ]
15. Klein RM (1979). Analysis of the intestinal cell proliferation after guanethidine-induced sympathectomy. II. Percentage labelled mitoses studies. Cell Tissue Kinet. 12: 649-657. [ Links ]
16. Klein RM, McKenzie JC (1980). Pattern of crypt cell proliferation in the pre- and post-closure ileum of the neonatal rat: effects of sympathectomy. Cell Tissue Res. 206: 387-394. [ Links ]
17. Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A (1976). Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Méd. 295: 417-420. [ Links ]
18. Kuyvenhoven JP, Meinders AE (1999). Oxidative stress and diabetes mellitus: pathogenesis of long term complications. Eur J Intern Med. 10: 9-19. [ Links ]
19. Lorenz-Meyer H, Thiel F, Menge H (1977). Structural and functional on the transformation of the intestinal mucosa in rats with experimental diabetes. Res Exp Med. 170: 89-99. [ Links ]
20. Miller DL, Hanson W, Schedl HP, Osborne JW (1977). Proliferation rate and transit time of mucosal cells in small intestine of the diabetic rat. Gastroenterology 73: 1326-1332. [ Links ]
21. Noda T, Iwakiri R, Fujimoto K, Yoshida T, Utsumi H, Sakata H, et al. (2001). Supression of apoptosis is responsible for increased thickness of intestinal mucosa in streptozotocin-induced diabetic rats. Metabolism. 50: 259-264. [ Links ]
22. Romano EB, Miranda-Neto MH, Cardoso RCS (1996). Preliminary investigation about the effects of streptozotocin-induced chronic diabetes on the nerve cell number and size of myenteric ganglia in rat colon. Rev Chil Anat. 14: 139-145. [ Links ]
23. See NA, Epstein ML, Dahl JL, Bass P (1990). The myenteric plexus regulates cell growth in rat jejunum. J Auton Nerv Sys. 31: 219-229. [ Links ]
24. Silva JM (2004). Effect of the autonomic extrinsic denervation on cellular dynamic of duodenal epithelium of the ault wistar mouse. Tese (Doutorado em Ciências - Biologia Celular e Molecular) - Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto. [ Links ]
25. Tutton PJ (1975). Neural stimulation of mitotic activity in the crypts of lieberkuhn in rat jejunum. Cell Tissue Kinet. 8: 259-266. [ Links ]
26. Vinson JA, Staretz ME, Bose P, Kassm HM, Basalyga BS (1989). In vitro and in vivo reduction of erythrocyte sorbitol by ascorbic acid. Diabetes 38: 1036-1041. [ Links ]
27. Will JC, Byers T (1996). Does diabetes mellitus increase the requirement for vitamin C? Nutr Rev. 54: 193-202. [ Links ]
28. Young IS, Torney JJ, Trimble ER (1992). The effect of ascorbate supplementation on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 13: 41-46. [ Links ]
29. Yue DK, McLennan S, Fisher E, Heffernan S, Capogreco C, Ross GR, et al. (1989). Ascorbic acid metabolism and polyol pathway in diabetes. Diabetes 38: 257-261. [ Links ]
30. Zanoni JN, de Miranda Neto MH, Bazotte RB, de Souza RR (1997). Morphological and quantitative analysis of the neurons of the myenteric plexus of the cecum of streptozotocin-induced diabetic rats. Arq Neuropsquiatr. 55: 696-702. [ Links ]
31. Zanoni JN, Hernandes L, Bazotte, RB, Miranda Neto MH (2002). Terminal ileum submucous plexus: Study of the VIP-ergic neurons of diabetes rats treated with ascorbic acid. Arq Neuropsiquiatr. 60: 32-37. [ Links ]
32. Zanoni JN, Buttow NC, Bazotte RB, Miranda Neto MH (2003). Evaluation of the population of NADPH-diaphorase-stained and myosin-V myenteric neurons in the ileum of chronically streptozotocin-diabetic rats treated with ascorbic acid. Auton Neurosci. 104: 32-38. [ Links ]
33. Zhao J, Yang J, Gregersen H (2003). Biomechanical and morphometric intestinal remodeling during experimental diabetes in rats. Diabetologia 46: 1688-1697. [ Links ]
34. Zoubi SA, Williams MD, Mayhew TM, Sparrow RA (1995a). Number and ultrastructure of epithelial cells in crypts and villi along the streptozotocin-diabetic small intestine: a quantitative study on the effects of insulin and aldose reductase inhibition. Virchows Arch. 427: 187-193. [ Links ]
35. Zoubi SA, Mayhew TM, Sparrow RA (1995b). The small intestine in experimental diabetes: cellular adaptation in crypts and villi at different longitudinal sites. Virchows Arch. 426: 501-507. [ Links ]
36. Zucoloto S, Diaz JA, Oliveira JS, Muccilo G, Sales Neto VN, Kajiwara JK (1988). Effect of chemical ablation of myenteric neurones on intestinal cell proliferation. Cell Tissue Kinet. 21: 213-219. [ Links ]
37. Zucoloto S, Silva JC, Oliveira JS, Muccilo G (1991). The chronological relationship between the thickening of smooth muscle, epithelial cell proliferation and myenteric neural denervation in the rat jejunum. Cell Prolif. 24: 15-20. [ Links ]
38. Zucoloto S, de Deus DA, Martins AA, Muglia VF, Kajiwara JK, Garcia SB (1997). The relationship between myenteric neuronal denervation, smooth muscle thickening and epithelial cell proliferation in the rat colon. Res Exp Méd. 197: 117-124. [ Links ]
Received on January 2, 2007.
Accepted on May, 2007