Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.32 n.2 Mendoza jun./ago. 2008
Rosette formation by macrophages with adhered T lymphocytes is precluded by inhibitors of antigen processing and presentation
Ivón Teresa Novak and Humberto Ramón Cabral
Instituto de Biología Celular, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, Av. Enrique Barros, Ciudad Universitaria, 5000 Córdoba, Argentina.
Address correspondence to: Dr. Humberto R.A. Cabral. Instituto de Biología Celular, Facultad de Ciencias Médicas, Universidad Nacional de Córdoba, ARGENTINA. E-mail: hcabral@fcm.unc.edu.ar
ABSTRACT: We had previously found in autologous human leukocyte cultures, in which dead neutrophils phagocytosis by macrophages occur, macrophages and T CD4 lymphocytes perform a selective cell-cell interaction showing many figures of either one, two or several T- lymphocytes adhering to a central macrophage were seen. Considering that antigen presentation would be necessary for the formation of these immune synapses, we attempted to block rosette formation (i.e., the formation of macrophage associations with at least three lymphocytes) by interfering with both antigen processing and presentation. Culture samples of autologous leukocytes from 7 healthy donors were subjected to either brefeldin A, chloroquine or to an anti- HLA DR antibody. Rosette formation was significantly inhibited in the treated samples (either with brefeldin A, chloroquine or the anti- HLA DR; ANOVA, p<0.001, as compared with the untreated controls). It is concluded that interference with antigen processing and presentation precludes the formation of these cellcell interactions.
Key words: Autologous rosetting; Cultured human macrophages and T- lymphocytes; Brefeldin A; Chloroquine; Immune synapses.
Introduction
One immune synapse is formed when a T lymphocyte adheres closely to an antigen-presenting cell (Grakoui et al., 1999; Lee et al., 2002; Dustin, 2005). We found that immune synapses occur in cultures of autologous human leukocytes, in which phagocytosis of dead neutrophils occurs, and in which rosettes of one macrophage surrounded by lymphocytes occur (Cabral and Novak ,1992). Single immune synapses or rosettes first appeared after 15 h in culture (Cabral and Novak, 1992, 1999), and this phenomenon was dependent on both time and cell density. Immunocytochemically, the lymphocytes surrounding macrophages were T CD4+ and they also positively reacted for UCHL-1, indicating that they were activated T-cells (Cabral and Novak, 1999).
We have previously observed that co-culture of both purified macrophages and lymphocytes did not induce rosette formation unless antigens were added to the culture medium (Cabral and Novak, 2003). We postulated, therefore, that antigen presentation would be involved in the formation of such immune synapses and rosettes in autologous leukocyte cultures, because an antigenic stimulus would derive from dead neutrophils in culture, whose materials (such as CD66 antigen) were identified inside monocyte-derived macrophages (Cabral and Novak, 1992, 1999; Novak and Cabral, 2002).
Insofar as cell-cell interactions in rosettes may correspond to an interaction of T CD4+ cells with class II major histocompatibility complex (MHC) molecules expressed by macrophages, the current study was launched to study the effects of brefeldin A and chloroquine (inhibitors of antigen-proccesing and presentation) as well as of a monoclonal antibody against anti-HLA molecules, respectively, on the immune synapse and rosette formation, considering that if antigen presentation is a necessary stimulus for the immune synapses and rosettes production.
Brefeldin A is a metabolite of the fungus Eupenicillium brefeldianum, which specifically and reversibly blocks protein transport from the endoplasmic reticulum to the Golgi apparatus (Klausner et al., 1992; Pelham, 1991), and was first described as a specific inhibitor of antigen processing for Class I-restricted T cell recognition (Yewdell and Bennink, 1989). However, currently, the inhibition of phagocytic antigen processing by brefeldin A has been described and correlated with depletion of nascent MHC-II molecules in phagosomes (Ramachandra and Harding, 2000). Chloroquine is known to increase the pH of endocytic vacuoles, both inhibiting antigen processing and presentation in the phagocytic pathway (Janeway et al., 2004). With respect to the use of anti-HLA DR, the idea was to block the HLA-DR molecule at the surface of the antigen presenting cells (the monocyte-derived macrophages).
Materials and Methods
Autologous Leukocyte Cultures
Blood samples were obtained from normal adult volunteers who gave their written informed consent (5 women, 4 men; mean age 32 years, range 18-58 years).Venous blood was obtained with sterile disposable syringes containing phenol-free heparin. Blood samples were allowed to sediment for 2 h. Plasma supernatants containing all types of leukocytes were separated and were cultured at 37ºC in TC199 medium (DIFCO, Detroit, Michigan, USA) supplemented with penicillinstreptomycin and 25% filtered autologous serum). Cell concentration was 300.000 leukocytes/mL. Culture samples were harvested with previous shaking of the culture flasks and centrifuged at 200 g and cytopreparations were made of each cell pellet, as follows: the cell pellets were gently dispersed in drops of TC 199 culture medium and distributed with Pasteur pipettes onto identified glass slides and left to settle for 5 min, in a humid chamber, and then desiccated by speedy rotation over a disk fixed on the axis of a centrifuge (Cabral and Novak, 1992, 1999). Control experiments were leukocyte cultures of the same donors, but in which brefeldin A, chloroquine or anti-HLA-DR were not added. Cell viability was assessed by the trypan blue exclusion test in all samples.
Cytopreparations were stained with either May-Grünwal-Giemsa, hematoxylin-eosin or toluidin blue and were studied under a bright field microscope. A macrophage surrounded either by one or two lymphocytes was called an immune synapse, while if it was surrounded by three or more lymphocytes was called a "rosette". The percentage of both immune synapses and rosettes in a given cytopreparation was computed after counting 100 macrophages.
Brefeldin A (SIGMA, St. Louis, MO) was added 4 and 7 h after the initiation of cell cultures and at either 5 µg/mL, 2.5 µg/mL or 1 µg/mL. In some experiments, the cells were washed with filtered autologous serum at 4 h after the addition of brefeldin A, they were centrifuged for 10 min at 200 g, and were cultured again in a medium without brefeldin A. Chloroquine (Rhône-Poulenc) was added at the initiation of cell cultures,to obtain a 0.1 mM concentration. The study was made at several times (after 24, 48, 72, 96, 120, and 144 h). Cell cultures without chloroquine were used as controls.
Anti-HLA
Venous blood was obtained from 4 human healthy donors. Autologous leukocyte cultures were performed as described above. A monoclonal antibody against HLA-DR (Diatec, Norway) was added to leukocyte cultures after 72 and 96 hours of culture, at a final concentration of 15 µg/mL, and was incubated for 30 minutes. Next, the technique for immune synapse and rosette expression was perfomed (Cabral and Novak, 1992, 1999). Cultures without anti-HLA-DR treatment were employed as controls. All samples were harvested and centrifuged at 200 g and prepared as described above.
Statistical analysis
Analysis of variance (ANOVA) and t test for paired differences were used for statistical evaluations.
Results
In the control experiments, rosettes were observed in cytopreparations from cell cultures after 15 hours. Figure 1A shows the harvested cells after 3 hours of culture, when nor rosettes were formed (Cabral and Novak, 1992, 1999), while figures 1B and 1C show rosettes formed at 48 and 72 hours of culture, respectively. The trypan blue exclusion test yielded values above 96% in all experiments.
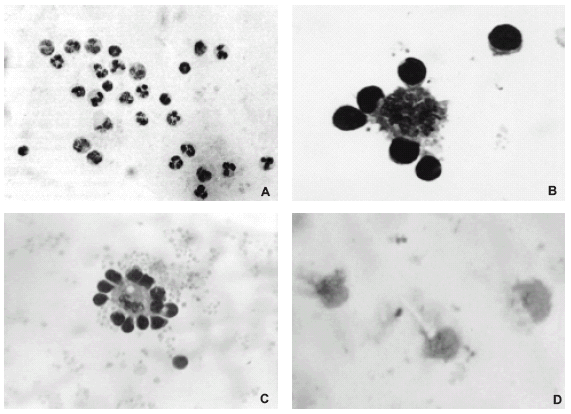
FIGURE 1. (A) Cytopreparation of culture from autologous human leukocytes obtained after 3 h of culture. Note that the cells are not forming any close association; hematoxylin-eosin, x200. (B) Rosette formed between a monocyte-derived macrophage and lymphocytes in a 48 h culture of leukocytes from a healthy subject; May-Grünwald-Giemsa, x400 and amplified. (C) Rosette formed between a human monocyte-derived macrophage and lymphocytes from a 72 h leukocyte culture; May-Grünwald-Giemsa, x400. (D) Leukocyte culture with brefeldin A at 5 µg/ml where no rosettes were formed; macrophages showed a spongy aspect; May-Grünwald-Giemsa, x400.
Experiments with BFA at 5 µg/mL
Rosettes were not formed when brefeldin A was added to these cell cultures (Fig. 2A). Monocytes already showed signs of their transformation into macrophages after 15 h of culture. After 48 h of culture with brefeldin A (Fig. 2A), macrophages showed a spongy aspect (Fig. 1D).
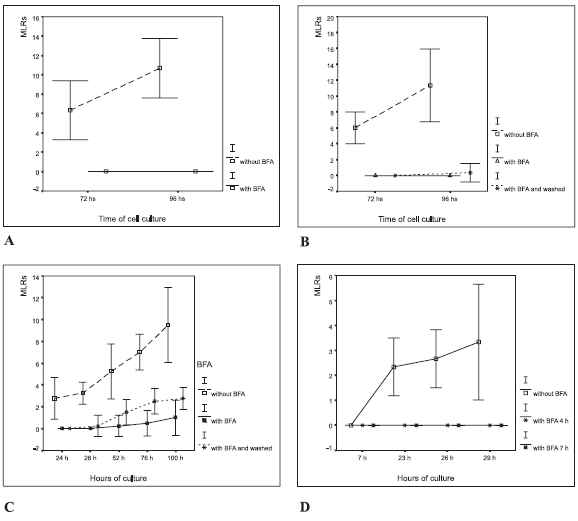
FIGURE 2. Macrophage-lymphocyte rosettes (MLR) in autologous leukocyte cultures with the addition of brefeldin A (BFA). The results are expressed as the percentage of the number of macrophages that formed rosettes in a given cytopreparation by counting 100 macrophages, (mean ± 2 SD). (A) 5 µg/mL in vitro; (B) 2.5 µg/mL in vitro and washed at 4 h. The ANOVA showed significant differences in rosette formation (p<0.0001) between treatments (BFA at 2.5 µg/ mL in vitro, and without BFA); (C) 1 µg/mL in vitro, at 4 and 7 h; (D) 1 µg/mL in vitro, and washed at 4 h.
Experiments with brefeldin A at 2.5 µg/mL and washed at 4 h
The effect of brefeldin A on cell cultures was reversed by washing with autologous serum after 4 h of culture (Fig. 2B). In such conditions, macrophages conserved their morphology after 72 h. Only a low number of macrophages had a single adhered lymphocyte after 96 h of culture.
Experiments with brefeldin A at 1 µg/mL, observed at 0, 4, and 7 h of culturing
Rosettes did not occur when brefeldin A was added to cell cultures at 0, 4 or 7 h, which contrasted with rosette formation in control cultures in which no brefeldin A was added (See Fig. 2C).
Experiments with brefeldin A at 1 µg/mL and washing at 4 h
Samples of leukocyte cultures with and without brefeldin A were harvested at 24 h, 28 h, 52 h, 76 h and 100 h (Fig. 2D). Significant differences in rosette formation were found (ANOVA, p<0.0001) between brefeldin A treated cultures and the controls. As expected, the number of immune synapses and rosettes increased in cultures that were washed at 4 h. These results showed that brefeldin A inhibits macrophage activity and rosette formation.
Effect of chloroquine on rosette formation
Inhibition of rosette formation was observed under chloroquine treatment (Fig. 3), which contrasted with rosette formation in the control cultures with no chloroquine added.
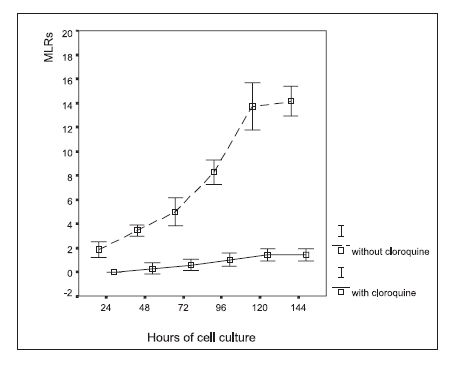
FIGURE 3. Macrophage-lymphocyte rosette (MLR) formation in autologous leukocyte cultures, with and without chloroquine (0.1 mM) treatment in vitro. The results are expressed as the percentage of the number of macrophages that formed rosettes in a given cytopreparation by counting 100 macrophages (mean ± 2 SD).
Effect of anti HLA-DR
The addition of anti HLA-DR to the cell cultures significantly inhibited the formation of both immune synapses and rosettes, both at 72 and 96 hours, as compared with the control cultures (Figs. 4 and 5; ANOVA, p<0.0001).
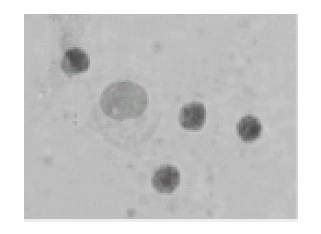
FIGURE 4. No rosettes nor immune synapses were formed in autologous leukocyte cultures treated with anti HLA-DR (96 h of culture); toluidin blue, x400.
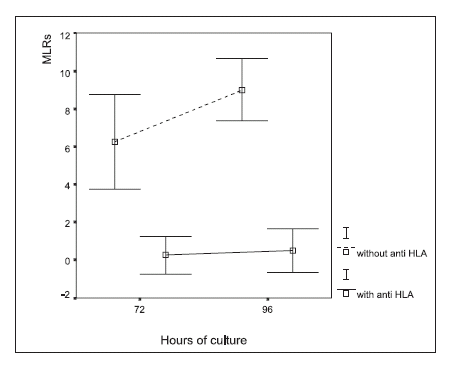
FIGURE 5. Macrophage-lymphocyte rosettes (MLR) in autologous leukocyte cultures treated with an anti HLA-DR monoclonal antibody. The results are expressed as the percentage of the number of macrophages that formed rosettes in a given cytopreparation by counting 100 macrophages, (mean ± 2 SD). ANOVA showed significant differences in rosette formation (p<0.0001) between treatments (with and without the addition of HLA-DR antibody)
Discussion
Macrophage-lymphocytic rosettes. i.e., the occurrence of several areas of close contact between a macrophage and several adhered T lymphocytes was found in previous work (Cabral and Novak, 1992, 1999) when cells were sampled from autologous leukocytes cultures. The central macrophage in them showed signs of phagocytosis of dead neutrophil materials. In the same cytopreparations many figures of one macrophage with only one or two adhered lymphocytes were found, thus, it would appear as an adequate model for studying features of the formation of both individual and multiple immune synapses between macrophages and T lymphocytes.
In a previous study (Cabral and Novak, 2003) we found that no rosettes were formed in co-cultures of purified, autologous monocytes and lymphocytes unless antigens were added to these cultures. In the current experimental model, in which antigens from dead neutrophils occur, both immune synapses and rosettes occurred 15 hours after the beginning of cultures, when blood monocytes showed morphological signs of macrophage transformation and have ingested materials from dead neutrophils (Cabral and Novak, 1992, 1999).
Moreover, we found that the number of rosettes increased from 15 to 120 hours of culture (Cabral and Novak (1992, 1999). The lymphocytes forming the rosettes are T-CD4 cells and were also positively reacting for CD45RO (clone UCHL-1), indicating that they are activated T-cells (Cabral and Novak, 1999).
Here, we have experimentally explored the formation of either single or multiple immune synapses and rosettes, after subjecting the cultured leukocytes to agents that could either prevent antigen-processing-presentation (as brefeldin A or chloroquine), or may act on the surface molecules of antigen presentation class II alone (antibodies anti HLA DR).
In recent studies, the inhibition of phagocytic antigen processing by brefeldin A have been described and correlated with depletion of nascent MHC-II molecules in phagosomes (Ramachandra and Harding, 2000). Our results would reinforce this concept. On the other hand, the finding that the number of rosettes increased in samples of cultures washed at 4 h, suggests the reversibility of effects of brefeldin A, as it was observed in other experimental models (Klausner et al., 1992; Pelham, 1991). In cell cultures with brefeldin A, macrophages showed morphological changes (Fig. 1D), also corroborating previous findings by other authors (Klausner et al., 1992; Pelham, 1991). We were interested in investigating the effects of brefeldin A at various times of cell culturing, as well as at several times of assays and conditions, because in this model, both rosettes and immune synapses are observed since 15 h of culturing, while brefeldin A effects have been reported in short- time assays only (Lippincontt-Schwartz et al., 1991; Hunziker et al., 1991). In our experiments, we found that a brefeldin A concentration of 1 µg/mL is a satisfactory concentration for these cellular studies.
Chloroquine has been described as an inhibitor of antigen processing and presentation in the phagocytic pathway, by increasing the pH of endocytic vacuoles (Janeway et al., 2004).
The results of this work showed that both brefeldin A and chloroquine inhibit the formation of immune synapses and autologous rosettes between macrophages and T lymphocytes. Exposure to an anti-HLA DR antibody also precluded the formation of immune synapses and rosettes. These results strongly suggest that both antigen processing and presentation are involved in the occurrence of immune synapses and rosettes. The effect of anti-HLA DR treatment, also suggests that such antibody can preclude the presentation at the molecular level, at the surface of the antigen processing cell. Hence, taken together, the current results support the hypothesis that antigen presentation would be a necessary stimulus for rosettes formation.
Acknowledgments
We thank Professor Martha Gonzalez Cremer for help in the preparation of the manuscript and Professor Silvia Joekes for statistical advice.
References
1. Cabral HRA, Novak ITC (1992). Spontaneous formation of rosettes by autologous human monocyte-macrophages and lymphocytes in cell cultures. Rev Fac Cienc Med Córdoba. 50: 25-26. [ Links ]
2. Cabral HRA, Novak ITC (1999). Autologous rosette formation by human blood monocyte-derived macrophages and lymphocytes. Am J Hematol. 60: 285-288. [ Links ]
3. Cabral HRA, Novak ITC (2003). Immunologic synapses and rosettes formation between human macrophages and lymphocytes stimulated with antigens in cell cultures. (abstract). Mol Biol Cell. 14:S, 458. ASCB. [ Links ]
4. Dustin ML (2005). A dynamic view of the immunological synapse. Semin Immunol. 17: 400-410. [ Links ]
5. Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin MI (1999). The immunological synapse: a molecular machine controlling T cell activation. Science. 285: 221-227. [ Links ]
6. Hunziker W, Whithney A, Mellman I (1991). Selective inhibition of transcytosis by Brefeldin A in MDCK cells. Cell. 67: 617-627. [ Links ]
7. Janeway CH, Travers P, Walport M, Shlomchik M (2004). Immunobiology: The Immune System in Health and Disease. Garland Science, New York, sixth edition, pp. 1-800. [ Links ]
8. Klausner RD, Donalson JG, Lippincott-Schwartz J (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 116: 1071-1080. [ Links ]
9. Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS (2002). T cell receptor signaling precedes immunological synapse formation. Science. 295: 1539-1542. [ Links ]
10. Lippincott-Schwartz J, Yuan L, Tipper CH, Amherdt M, Orci L, Klausner RD (1991). Brefeldin A'effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 67: 601-616. [ Links ]
11. Novak ITC, Cabral HRA (2002). CD66 monoclonal antibodies recognize neutrophil material phagocytized by macrophages that form immunological synapses and rosettes with T-lymphocytes. (abstract). Mol Biol Cell. 13:S, 493. ASCB. [ Links ]
12. Pelham HRB (1991). Multiple targets for brefeldin A. Cell. 67: 449-451. [ Links ]
13. Ramachandra L, Harding CV (2000). Phagosomes acquire nascent and recycling class II MHC molecules but primarily use nascent molecules in phagocytic antigen processing. J Immunol. 164: 5103-5112. [ Links ]
14. Yewdell JW, Bennink JR (1989). Brefeldin A specially inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 244: 1072-1075. [ Links ]
Received on May 20, 2007.
Accepted on August 17, 2007.














