Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.32 n.2 Mendoza jun./ago. 2008
Chromosome comparison between populations of the collared peccary, Tayassu tajacu, raised in captivity
Patrícia Carvalho de Souza, André Salim Khayat, Igor Chamon Seligmann and Rommel Mario Rodríguez Burbano
Laboratório de Citogenética Humana, Centro de Ciências Biológicas, Universidade Federal do Pará, Belém, PA, Brazil.
Address correspondence to: Rommel Burbano. Laboratório de Citogenética Humana, CCB, Universidade Federal do Pará, Campus Universitário do Guamá, Rua Augusto Corrêa 01. CEP 66075-110. Belém. PA, BRASIL. E-mail: rommel@ufpa.br
ABSTRACT: The collared peccary (Tayassu tajacu) is widely distributed over the American continent, being found from the south of the USA to the north of Argentina. In Brazil, it is spread all over the country, being one of the potential species to be raised in captivity. Therefore, the cytogenetic techniques could be a potential tool for reproductive monitoring of animals raised in captivity, mainly when destined for commercial purposes. This study had the objective of determining the chromosome number of two populations raised in captivity and characterizing them by GTG banding. For this purpose, an analysis was made of mitotic metaphases obtained from lymphocyte cultures made from blood samples of 11 animals, six of which from the Northeast and five from the North of Brazil. The results of this analysis showed the same karyotype pattern for the species (2n=30 chromosomes and NF=48), besides corresponding to the South American pattern of the species, i.e., without a translocation between autosomes 1 and 8, chromosome X acrocentric, and no differences were found between the two populations studied. However, chromosomal polymorphisms were observed compared to data from the literature on populations from North and South America.
Key words: Cytogenetics; GTG Banding; Tayassuidae; Suidae; Pigs
Introduction
Most of the wild Suidae have already been studied karyotypically. According to Bosma et al. (2004), the differences between the karyotypes of these species consist mainly of Robertsonian translocations and varying quantities of centromeric heterochromatin. The relative homogeneity among the karyotypes of the extant Suidae is in contrast with the wide karyotype variation observed among the Tayassuidae. Plausible explanations address the possibility that the three existing species of Tayassuidae are phylogenetically less closely related than usually assumed, or that their genome is less stable than that of Suidae, and that chromosome rearrangements were much more frequent during the evolution of this family as compared to Suidae (Bosma et al., 2004).
Although T. tajacu is widely spread over the American continent, as it is found from the southern US to the north of Argentine (Giannoni, 1973; Giannoni et al., 1981; Grubb and Groves, 1993; Sowls, 1997), being actually the most widespread species of Tayassuidae, there are only few reports on the chromosomal constitution of this species.
In Brazil, where the species is widespread, a karyotype characterization of different groups coming from different regions and raised in captivity would favor the knowledge of possible chromosome polymorphisms which might compromise the reproduction and the phenotype of the species, also serving as a tool to help in the management of animals raised in captivity for commercial purposes. Thus, the objective of the present study was to determine the chromosome number of T. tajacu from the Brazilian North and Northeast raised in captivity, and to characterize it by GTG banding.
Material and Methods
Eleven adult animals, the matrixes of the breeding stock of the Álvaro Adolfo Experimental Field of the Empresa Brasileira de Pesquisa Agropecuária - EMBRAPA - Eastern Amazon, were used. Five came from northern Brazil and six from the Brazilian Northeast.
From 3 to 5 mL of peripheral blood were drawn from the brachial vein of the front leg, previously submitted to local asepsis with 70% alcohol iodine, using syringes that had been previously heparinized. Lymphocytes were obtained from cultures as described by Moorhead et al. (1960), with modifications.
Slides were submitted to conventional staining (Giemsa 1:30) and, after one week of aging, the GTG banding technique was performed (Scheres, 1972, and Seabright, 1971), with modifications. For each animal, 100 metaphases were analyzed and the chromosomes were identified and classified according to Andrea et al. (2001) and Bosma et al. (2004). Photomicrographs of the best metaphases were made under an optical microscope (Nikon Labophot-2) with 100X magnification, for karyotype mounting.
Results and Discussion
All animals studied (n=11), regardless of their diverse origins, presented the same chromosomal constitution (2n=30 and NF=48), consistent with the results found by Krallinger (1936), Spalding and Berry (1955), Giannoni (1972, 1973), Giannoni and Ferrari (1976a, b, c, d), Santos et al. (1995), Bosma et al. (2004), Puertas et al. (2004) and Adega et al. (2006), reinforcing this chromosomal constitution as being characteristic of the species (Fig. 1a).

FIGURE 1. a) G-banded karyotypes of a male of species T. tajacu. b) Idiogram of the sex chromosomes.
The autosomes were morphologically classified and divided into two different groups, according to Andrea et al. (2001) and Bosma et al. (2004): meta/submetacentrics (pairs 01-07) and acro/telocentrics (pairs 08-14), as shown in figure 1a and Table 1.
TABLE 1. Morphology of autosomes and sex chromosomes in the karyotype of T. tajacu.
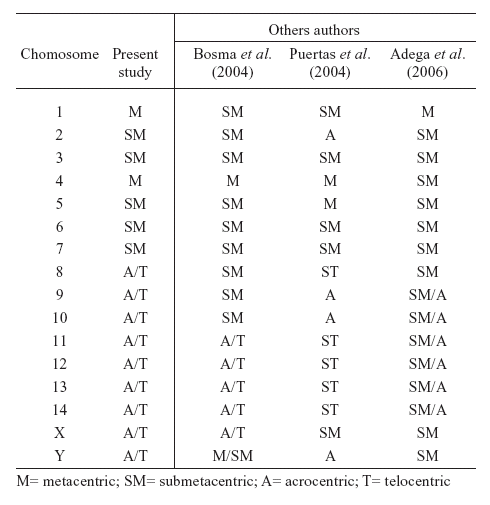
According to their GTG banding pattern (Fig. 1a), showing absence of the translocation between chromosomes 1 e 8, that is characteristic for North and Central American species, as observed by Adega et al. (2006). The two populations studied can be classified as South American representatives of the species, a pattern determined by Puertas et al. (2004).
Considerable differences in size were observed between the largest pair of autosomes (pair 01) and the smallest ones (pairs 13 and 14) of the karyotype. The X chromosome was slightly larger in size than chromosome 8, the largest acro/telocentric autosome in the karyotype. However, the Y chromosome was the smallest of all chromosomes.
In this sample, the chromosome X and Y showed acrocentric morphology (Fig. 1b), a pattern which is not present in the North American representatives of T. tajacu (Adega et al., 2006). The acro/telocentric X chromosome was also described in the chromosome constitution of a South American couple of T. tajacu by Bosma et al. (2004) (Table 1) and, together with the translocation between chromosomes 1 e 8, would be useful as a marker suitable for sub-species differentiation.
We suggest that, to carry out a quantitative analysis of the species, it should be enough to search for the presence or absence of these chromosome markers. Thus, exclusively in the case of species T. tajacu, cytogenetics becomes a rapid and efficient tool for characterizing the origin of the breeding stock.
Acknowledgements
This work was supported by Financiadora de Estudos e Projetos (FINEP CT-INFRA/FADESP) grant No. 0927-03. RRB has PQ-2 fellowship (number 308256/2006-9) granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
1. Andrea MV, Oliveira C, Rocha GT, Foresti F (2001). Cytogenetical and histological studies in testis of Tayassu tajacu and a natural interspecific hybrid. J Anim Breed Genet. 118: 125-133. [ Links ]
2. Adega F, Chaves R, Kofler A, Krausman PR, Masabanda J, Wienberg J, Guedes-Pinto H (2006). High-resolution comparative painting in the Arizona collared peccary (Pecari tajacu, Tayassuidae): a comparison with the karyotype of pig and sheep. Chromosome Res. 14: 243-251. [ Links ]
3. Bosma AA, De Haan NA, Arkesteijn GJA, Yang F, Yerle M, Zijlstra C (2004). Comparative chromosome painting between the domestic pig (Sus scrofa) and two species of peccary, the collared peccary (Tayassu tajacu) and the white-lipped peccary (T. pecari): A phylogenetic perspective. Cytogenet Genome Res. 105: 115-121. [ Links ]
4. Giannoni MA (1972). Os cariótipos das espécies Tayassu tajacu e Tayassu albirostris. In: 24 ª Reunião da Sociedade Brasileira para o Progresso da Ciência: Resumos, São Paulo, p. 71. [ Links ]
5. Giannoni MA (1973). Estudo cariotípico de alguns representantes da Família Suidae. Dissertação de Mestrado, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo, Ribeirão Preto, pp. 130. [ Links ]
6. Giannoni MA, Ferrari I (1976a). Padrões de Formação de Gbandas dos Cromossomos da Espécie Tayassu tajacu. I-Análise dos Pares Cromossômicos Números 1 a 7. Científica. 4(1): 72-77. [ Links ]
7. Giannoni MA, Ferrari I (1976b). Padrões de formação de G-bandas dos cromossomos da espécie Tayassu tajacu. I - Análise dos pares cromossômicos números 8 a 15. Científica. 4(1): 78-81. [ Links ]
8. Giannoni MA, Ferrari I (1976c). Inversão, translocação e a provável formação do par cromossômico número 1 da espécie Tayassu tajacu. Ciência e Cultura. 28(10): 1160-1164. [ Links ]
9. Giannoni MA, Ferrari I (1976d). Inversão, fusão Robertsoniana e a provável formação do par cromossômico número 2 da espécie Tayassu tajacu. Ciência e Cultura. 28(10): 1164-1169. [ Links ]
10. Giannoni MA, Ferrari I, Giannoni ML (1981). C-banding patterns in the chromosomes of the subspecies Sus scrofa scrofa (wild pig) and Sus scrofa (domestic pig). Revista Brasileira de Genética. IV (3): 399-410. [ Links ]
11. Grubb P, Groves CP (1993). Chapter 2: The Neotropical Peccaries - Dicotylidae: Tayassu and Catagonus. In: Status Survey and Conservation Action Plan - Pigs, Peccaries and Hippos, W.L.R. Oliver, Ed. IUCN. http://www.iucn.org/themes/ssc/sgs/pphsg/APchap2-1.htm [ Links ]
12. Krallinger HF (1936). Die Chromosomen des Halsbandpekaris (Pecari tajacu). Z Zellf Mikr Anat. 24: 1-10. [ Links ]
13. Moorhead PS, Nowell PC, Mellman JW, Battips DM, Hungerford DA (1960). Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 20: 613-616.b [ Links ]
14. Puertas DF, Díaz ID, Ortiz JBL (2004). Evidencia de estructuración cromosómica asociada con la distribución geográfica de Pecari de Collar (Tayassu tajacu) de Centro y Suramérica. In: VI Congreso Internacional sobre Manejo de fauna Silvestre en la Amazônia y Latinoamérica: Libro de Resúmenes. Iquitos - Peru. [ Links ]
15. Santos PA, Rocha GT, Lucca EJ (1995). Análise citogenética da espécie Tayassu tajacu (Cateto - Mammalia). J Genetics. 18 (suppl.3): 495. [ Links ]
16. Seabright M (1971). A rapid technique for human chromosomes. Lancet. 2: 971-972. [ Links ]
17. Scheres JM.JC. (1972). Identification of two Robertsonian translocations with a Giemsa banding technique. Humangenetik. 15: 253-256. [ Links ]
18. Sowls LK (1997). Javelinas and other Peccaries - Their biology, management and use. 2nd ed., Eds. Texas A&M University Press, College Station, Texas, pp. 352. [ Links ]
19. Spalding JF, Berry RO (1955). A chromosome study of the wild pig (Pecari angulatus) and the domestic pig (Sus scrofa). Cytologia. 21: 81-84. [ Links ]
Received on November 27, 2007.
Accepted on May 7, 2008.














