Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.32 n.3 Mendoza sep./dic. 2008
Isolation of epithelial cells, villi and crypts from small intestine of pigeons (Columba livia)
Oscar Mac Donal1, Juan G. Chediack1,2,3 and Enrique Caviedes-Vidal1,2,3
1. Laboratorio de Biología "Prof. Enrique Caviedes-Codelia". Facultad de Ciencias Humanas. Universidad Nacional de San Luis - CONICET. Casilla de Correos 226. (5700) San Luis, Argentina.
2. Área de Biología. Departamento de Bioquímica y Ciencias Biológicas. Facultad de Química, Bioquímica y Farmacia. Universidad Nacional de San Luis. Chacabuco 917. (5700) San Luis, Argentina.
3. Instituto Multidisciplinario de Investigaciones Biológicas (IMIBIO-SL-CONICET) UNSL. Ejército de los Andes 950. (5700) San Luis, Argentina.
Address correspondence to: Juan G. Chediack. Laboratorio de Biología «Prof. Enrique Caviedes-Codelia». Facultad de Ciencias Humanas, Universidad Nacional de San Luis. Casilla de Correos 226, (5700) San Luis, ARGENTINA. Fax: (+54 2652) 430224. E-mail: jg.chediack@gmail.com
ABSTRACT: The isolation of viable enterocytes, villi and crypts from the small intestine of a feral bird (Columba livia) is important for performing physiological experiments in ecologically relevant processes of membrane transport. The effectiveness of mechanical disruption, enzymatic digestion and chelating agents were compared. The objectives were to isolate enterocytes, villi and crypts from the small intestine of young pigeons; to evaluate the viability of the isolated intestinal epithelial cells isolated; and to verify the integrity of enterocytes by biochemical features. Enzymatic and mechanical methods yielded both elongated columnar and spherical cells. With the chelating method villi and crypts were obtained. All methods produced a high yield of intestinal epithelial cells with about 50 % viability. Brush border enzymes (sucrase-isomaltase and alkaline phosphatase) activities were high and, as reported in chickens, they did not differ along the intestinal villus-crypt axis. Although the three methods have good viabilities, the enzymatic technique gives the best yield in cell number, while the chelating method provides the highest populations of morphologically distinctive villi and crypts.
Keywords: Enterocyte; Villus; Crypt; Isolation methods; Avian gut
Introduction
The isolation of intestinal epithelial cells, villi and crypts is particularly important for studies of digestive physiology including developmental physiology (Traber, 1999; Uni et al., 1997; King et al., 2000; Uni et al., 2003), membrane transport processes (Audus et al., 1990; Garriga et al., 1997; Artursson and Borchardt, 1997; Wolffram et al., 1998; Angelo et al., 2002), digestive enzyme activity in different maturation stages of the enterocyte (Traber, 1990; Ferraris et al., 1992; Fan and Stoll, 2001; Uni et al., 1998; Semenza et al., 2001) and cellular biotransformation of agrochemicals (Kurihara et al., 1993). In addition, the availability of freshly isolated intestinal epithelial cells and crypts opens the possibility of using relatively undifferentiated enterocytes as starting material for primary cultures and generation of cell lines (Quaroni et al., 1979; Perreault and Beaulieu, 1998; Velge et al., 2002; Weng et al., 2005).
Mammalian intestinal cell isolation for cell culture has been more extensively studied than avian intestinal cell isolation for the same purpose (Quaroni and May, 1980; Booth and O'Shea, 2002; Kaeffer, 2002). Moreover, most avian intestinal cell studies were performed in chickens (Uni et al., 1998; Angelo et al., 2002; Velge et al., 2002) while we are interested in obtaining intestinal cells from a feral bird (Columba livia) for studies on intestinal membrane transport processes with ecological relevance (Caviedes-Vidal, 2003).
The mucosa of the small intestine is arranged into two fundamental structures: villus and crypt. Villi are projections into the lumen covered predominantly with mature, absorptive enterocytes while crypts (of Lieberkhün) are moat-like invaginations of the epithelium around the villi. Stem cells found at the base of the crypts continually divide and provide the source of all the epithelial cells in the crypts and on the villi (Booth and O'Shea, 2002).
Isolation of cells from tissue requires disruption of the cellular junctions by mechanical, enzymatic or chelating methods. There are different techniques for the preparation of isolated intestinal cells by these methods, which provide a large amount of cells and usually allow an adequate separation between villi and crypts (Quaroni and May 1980).
The first objective of this study was the sequential isolation of enterocytes, villi and crypts from the small intestine of young pigeons utilizing shaking, enzymes and chelating agents, and their microscopic examination. The second objective was to compare the viability of the cells obtained with the three classical methods: mechanical (shaking), enzymatic (trypsin in low concentration) and a chelating agent (EDTA). The third objective was to verify the integrity of enterocytes by biochemical features, measuring the specific activities of brush-border digestive enzymes (sucrase-isomaltase and alkaline phosphatase).
Materials and Methods
Materials
Trypsin-EDTA 0.05% was obtained from HyClone (Logan, Utah, USA). Trypan Blue was provided by BioPack (Buenos Aires, Argentina). Glucose and alkaline phosphate detection kits were obtained from Wiener Laboratorios SAIC (Rosario, Argentina). All other reagents were purchased from Anedra S.A. (Buenos Aires, Argentina) and Sigma Chemical Co. (St. Louis, MO, USA).
Animals and sample collection
Fifteen young feral pigeons (Columba livia; Linneo, 1758), five for each treatment, were captured from a nest near the Universidad Nacional de San Luis Campus (San Luis, Argentina) and carried to the laboratory for trials. The birds were anesthetized using ethyl ether, the abdominal cavity was opened and the entire gastrointestinal tract was removed and chilled in ice-cold Hanks' balanced salt solution with mannitol (HBSSmannitol). The content of the small intestine (SI) was removed, and the SI was cleaned of extraneous tissue, measured for length and weighed. The SI was divided in three parts, proximal (closer to the pylorus) medial and distal. The medial segment was used in the experiments because this part has a high digestive activity (Ciminari et al., 2005). The medial portion of the SI was divided longitudinally into two parts and weighed. One part was destined to the isolation experiment and the other part was immediately frozen to -140ºC and stored for further enzymatic analysis.
Methods of enterocyte isolation
Mechanical shaking:
The intestinal segment from the medial section was washed with ice-cold HBSS-mannitol and cut into small pieces before isolation. The epithelial cells from the crypt-villus axis were isolated by a modification of the method previously described (Velge et al., 2002; Booth and O'Shea, 2002), with mincing and stirring with a magnetic bar at 500 rpm before shaking. In our case, two aliquots of cells were separated at room temperature by vigorous shaking (with vortex) for one minute in HBSS-mannitol. To obtain the first fraction, the tissue underwent one-minute vigorous shaking with vortex, and then the cells were separated from the rest of tissue by filtration through a sieve. The remaining tissue was subjected to another minute of shaking to obtain the second fraction of cells, which were then separated by filtration through a sieve. Dislodged cells in each fraction were harvested.
Enzymatic separation by using low trypsin concentration:
The intestinal segment was washed with chilled HBSS-mannitol before isolation. The epithelial cells from the small intestine were isolated by a modification of the method described by Eade et al. (1981) and Booth and O'Shea (2002), (both used 0.25% trypsin). Briefly, the medial portion of the small intestine was cut into five 0.5-cm long segments and incubated in an oven at 37ºC for 30 min in 5 ml of 0.05% Trypsin - EDTA (0.5 g of 1:250/L Porcine Trypsin in HBSS with 1.5 mM EDTA, pH 7.6-8.0) and then the cells were separated from the rest of the tissue by filtration through a sieve. The remaining tissue underwent 30 min of incubation in an oven at 37ºC with fresh enzymatic solution to obtain the second fraction of cells, which were then separated from the rest of the tissue by filtration through a sieve. The epithelial cells from both fractions were harvested.
Cell isolation by using chelating agents:
The intestinal segment from the medial section was washed with chilled HBSS-mannitol and cut into small pieces before isolation. The epithelium from the cryptvillus axis was isolated by a modification of the technique reported by Walters (1993) and Booth and O'Shea (2002). Villi were obtained by incubating the segments in 3 mM EDTA with 2 mM dithiothreitol in isotonic solution at 4ºC for one hour, followed by gentle shaking for few seconds. The rest of the tissue was placed in a new dish and fresh chelating solution was added before incubating the tissue at 4ºC for one hour. For isolation of crypts, the remaining tissue underwent an additional 1-hour incubation, which was followed by gentle shaking for few seconds.
Harvesting of cells
The cell fractions were washed three times with HBSS-mannitol by centrifugation at 75 g for 5 min at 4ºC, resuspended in 1 ml of HBSS-mannitol, and dispersed by several passages through a needle prior to cell counting (except for chelating method).
Viability tests
Cell integrity was tested by the method of Trypan blue exclusion (0.4% in physiological saline solution), a method based on the assumption that live cells are impermeable to the dye. Immediately after isolation, cells were counted using a Neubauer chamber.
Enzyme assays
Sample preparation
Intestinal segments (the medial portion without disaggregation and the other used for cell fractioning) or cell fractions were thawed at 4ºC, resuspended in 350 mM mannitol in 1 mM Hepes/KOH buffer (pH 7) (10 ml/g tissue), and homogenized for 30 s using a Fisher Scientific homogenizer (Ciminari et al., 2005). Enzyme activities in these fractions were determined as indicated below.
Intestinal enzyme activities
The activities of two digestive enzymes from the brush border of enterocytes were assayed in homogenates from both isolated fractions of epithelial cells and the portion of the same part of the intestine without disaggregation (see above in animal and sample collection). To determine the activity of the disaccharidase sucrase- isomaltase (E.C. 3.2.1.48) the colorimetric method developed by Dahlqvist (1984) and modified by Martinez del Río (1990) was used. Aliquots of 40 μl of tissue or cell homogenate, appropriately diluted, were incubated with 40 μl of 56 mM sucrose solution in 0.1 M maleate/NaOH pH 6.5. After 10 min incubation at al 40ºC the reaction was stopped by adding 1 ml of Glicemia Enzimática reagent (Wiener Laboratorios SAIC, Rosario-Argentina, with a sensitivity < 10 μg/ ml). The enzymatic activity was determined by measuring at room temperature the absorbance at 505 nm with a Spectronic 21D spectrophotometer.
Alkaline phosphatase (E.C. 3.1.3.1) activity was determined spectrophotometrically by the rate of sodium phenyl phosphate hydrolysis using an alkaline phosphatase kit (Wiener Laboratorios SAIC, Rosario-Argentina) following the manufacturer's recommendations. Briefly, aliquots of 40 μl of tissue or cell homogenate, appropriately diluted, were incubated with 40 μl of developing reagent (sodium phenyl phosphate in aminomethyl propanol alkaline buffer). Hydrolyzed phenol was detected after a 10-min incubation with 4-aminoantipyrine and ferricyanide at 40ºC. The sensitivity of this method is 10-3 units/ml. The samples were read immediately in a Spectronic 21D spectrophotometer at 520 nm.
Protein measurement
The protein concentration of samples was estimated by Lowry's method (1951) using the commercial kit Proti2 Assay (EDTA/Cu Reagent, Wiener Laboratorios SAIC, Rosario-Argentina). Absorbance was read at 540 nm using bovine serum albumin as the standard, with a sensitivity of 20 μg/ml.
Microphotographs of intestinal epithelial cells
Microphotographs of cells fractions, villi and crypts were taken using an Olympus microscope model BX50 and video camera Sony CCD-Iris. For measurements of lengths and widths of isolated enterocytes, the freeware software ImageJ (Rasband, 1997-2006) was employed.
Statistical analysis
Results are given as means ± 1 SEM; n is number of individuals. Standard least-squares methods were used to estimate parameters of linear regression. A Student's t-test was used to assess the statistical difference of enzyme activities and protein content between different fractions of isolated cells. One way analysis of variance (ANOVA) was used to compare the viability of cells obtained with enzymatic and mechanical methods. The significance level was set at P < 0.05.
Results
Microscopic appearance of isolated epithelial cells
All three methods allowed isolation of morphologically identifiable intestinal epithelial cells. In both fractions, the enzymatic and the mechanical method yielded both elongated columnar and spherical cells. The columnar cells are typically from the villus tip and the spherical ones from mid- and lower villus (Hartmann et al., 1982). Cells occurred singly and in clusters (Figs. 1 and 2). The first fraction of the chelating method allowed obtaining mainly villus units or portions of villi (Fig. 3A) while the second fraction produced crypts or portions of crypts (Fig. 3B). In all cases, the suspensions of isolated intestinal epithelial cells were minimally contaminated by mesenchymal and plasma elements.

FIGURE 1. Cell isolation by enzymatic method. A) Columnar and spherical cells isolated after 30-min incubation with 0.05% trypsin-EDTA, B) Spherical cells isolated after 60-min incubation with trypsin 0.05%.

FIGURE 2. Cell isolation by mechanical method. A) cluster of cells isolated after 1 min of shaking with vortex, B) clusters of cells isolated after 2 min of shaking with vortex.

FIGURE 3. Cell isolation by chemical method. A) Units of villus isolated after 1-hour incubation in an EDTA solution, B) Units of crypts isolated after two hours in EDTA solution.
Intestinal epithelial cell isolation
The dimensions of isolated pigeon jejunal enterocytes were: for columnar cells (Fig. 1; n = 10), 49.19 ± 1.1 μm length and 24.86 ± 0.81 μm of diameter, and for spherical cells (Fig. 1; n = 50) 33.41 ± 0.67 μm of diameter.
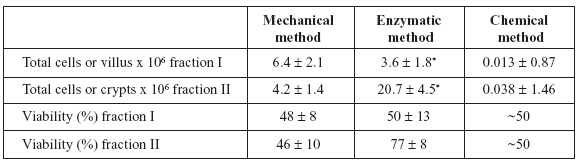
Cell counts and viabilities for mechanical and enzymatic methods and the yield of villi (or at least portions of villi) and crypts for the chelating method are shown in Table 1. The viability of the chelating method is an approximation, because we analyzed units or major parts of villi or crypts. There are no significant differences between methods in viability and number of cells obtained in both fractions, except for the enzymatic method (P < 0.032).
TABLE 1. Yields (in number of cells, villus or crypts per ml) and viabilities of intestinal epithelial cell isolates from pigeons, separated by mechanical, enzymatic and chelating methods. Values are means ± SEM (n=5). * P < 0.032.
Protein content
The protein concentration (mg per gram of tissue) in the cells isolated by the three different methods was not statistical different between each fraction with any method: 48.9 ± 2.3 vs 50.2 ± 6.5 (P > 0.64) for the mechanical method, 56.6 ± 7.8 vs. 61.9 ± 4.9 (P > 0.54) for the enzymatic method and 48.69 ± 4.2 vs 50.75± 4.1 (P > 0.84) for the chelating method. The ANOVA comparison for all methods gives no difference in protein content (P > 0.32).
Levels of brush border enzyme activity
Brush border enzyme activities (sucrase-isomaltase and alkaline phosphatase) of intestinal cells were determined, in both cell fractions, after isolating cells by enzymatic, mechanical and chemical methods. The activities of sucrase-isomaltase and alkaline phosphatase were high in both fractions of cells and they represent around 75% of the activities of the whole tissue used for comparison (whole intestinal segment - see animal and sample collection in materials and methods) (Tables 2 and 3). However, these differences were not statistically significant (all P > 0.10).
TABLE 2. Specific activity of sucrose-isomaltase (μmol·min-1·g-1 protein) in whole tissue and cells fractions. Values are means ± SEM (n=5).

TABLE 3. Specific activity of alkaline Phosphatase (UI phenol·g-1 protein) in whole tissue and cells fractions. Values are means ± SEM (n=5).

Discussion
The small intestinal epithelium constitutes a system in constant renewal. Enterocytes comprise up to 90% of epithelial cells in the crypt and over 95% of villus cells (Cheng and Leblond, 1974). The typical enterocyte from the villus-tip is recognizable under light microscopy by its oblong shape and conspicuous brush border (Fig. 1B). The spherical cells are typical for midand lower villus and crypts (Fig. 1A; Hartmann et al., 1982). In the mechanical and enzymatic methods, spherical cells constituted the main cell type isolated in both cell fractions (Figs. 1 and 2).
An interesting point of the present results concerns the dimensions of isolated pigeon jejunal enterocytes where columnar cells, typical for villus-tip, were 49.19 ± 1.1 μm in length and 24.86 ± 0.81 μm in diameter. These dimensions are larger than those of enterocytes isolated from the jejunum of rat and fish, but agree with dimensions of duodenal enterocytes obtained from young chickens and reptiles (Table 4).
TABLE 4. Dimensions of enterocytes in different taxa.

The differences in size have significance in aspects like absorption and digestion, because a large surface area may enhance nutrient digestion and uptake. On the other hand, a smaller number of enterocytes per unit of intestinal surface area might be associated with fewer cell junctions and, therefore, with fewer potential paths for paracellular absorption.
Suspensions of isolated intestinal epithelial cells, minimally contaminated by non-epithelial elements are relatively easy to obtain. However, there is controversy about the viabilities of cells after different mechanical, enzymatic and chelating methods (Eade et al., 1981; Ferraris et al., 1992; Uni et al., 1998). In this study, high viabilities of cells (see Table 1) were found with all methods. Moreover a better result in cell number and viability was shown for the enzymatic technique with a low concentration of trypsin in the second fraction. The chemical method with chelating agents provided enriched populations of morphologically distinctive villi and crypt units, which are free from contamination by non-epithelial elements, such as fibroblasts. The advantage of the chelating method was that crypts were easily obtained and the isolated crypts appeared to maintain their structure and proliferative potential. This is remarkable since crypts are widely used in digestive research (Pothier and Hugon, 1980; Ferraris et al., 1992; Uni et al., 1998; Fan et al., 2001; Angelo et al., 2002; Weng et al., 2005).
Four approaches are available to study the differentiation-dependent expression of enterocyte function in vivo: serial sectioning technique, quantitative cytochemical analysis, quantitative immunohistochemical analysis, and sequential cell isolation in combination with biochemical analysis (Smith, 1985). In the present case, the serial sectioning, cytochemical analysis and immunohistochemical analysis techniques are difficult to quantify and limited by availability of specific antibodies. Sequential cell isolation in combination with biochemical assays enables kinetic analyses of digestive enzyme activity in enterocytes under various conditions. This approach has been used previously in rabbits and rodents (Raul et al., 1977; Rowling and Sepulveda, 1984).
In mammals, cells in the enterocyte lineage divide several times as they migrate up the crypts. As they migrate towards the villus, they differentiate further, expressing all the transport proteins and enzymes characteristic of absorptive cells (Weiser, 1973; Traber, 1999). Hence, decreasing activities of alkaline phosphatase and sucrase-isomaltase along villus-crypt axis have been described in mammals (Pothier and Hugon, 1980; Ferraris et al., 1992; Fan et al., 2001). This contrasts with the lack of clear definition of the zones of enterocyte differentiation in chicken (Uni et al., 1998) which indicates that in this case further proliferation of enterocytes takes place after they leave the crypt. Consequently, the activity of sucrase-isomaltase and alkaline phosphatase shows high levels of activity along the crypt-villus tip axis, but alkaline phosphatase decreases in crypts (Uni et al., 1998). In pigeons high activities of sucrase-isomaltase and alkaline phosphatase were here found with all methods for both fractions, although as already stated, the chemical method yields mostly crypts (see Fig. 3). These results agree with those reported by Uni et al. (1998) in that, in avian intestine, enterocyte differentiation is not precisely localized. In contrast with Eade et al. (1981) who recommend careful selection of a method for cell isolation because some methods (e.g. mechanical and enzymatic) could affect the integrity of some proteins of the cellular surface, no differences between brush border sucrase-isomaltase and alkaline phosphatase activities in the whole tissue and the isolated enterocytes were found in the present work.
The isolation of viable enterocytes, villi and crypts from the small intestine of a feral bird raises the possibility of using relatively undifferentiated enterocytes as the starting material for cell culture. It also allows physiological experiments in membrane transport processes with ecological relevance.
Acknowledgements
We thank Fabricio Cid for their help in statistical analysis and M.E Ciminari, G. Moyano and R. Doña for their logistic support in the laboratory. We thank Sergio Alvarez for his help in the English edition. This work was supported by FONCYT (PICT 2004 - 25561) and CYT-UNSL (22/Q751) to Enrique Caviedes-Vidal.
References
1. Angelo S, Rojas AM, Ramirez H, Deves R (2002). Epithelial cells isolated from chicken jejunum: an experimental model for the study of the functional properties of amino acid transport system b(0,+). Comp Biochem Physiol A Mol Integr Physiol. 132(3): 637-644. [ Links ]
2. Artursson P, Borchardt RT (1997). Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm Res. 14(12): 1655-1658. [ Links ]
3. Audus KL, Bartel RL, Hidalgo IJ, Borchardt RT (1990). The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm Res. 7: 435-451. [ Links ]
4. Booth C, O'Shea J (2002). Isolation and Culture of Intestinal Epithelial Cells. In: Culture of Epithelial Cells. Second Edition. R. Ian Freshney and Mary G. Freshney Eds. Wiley-Liss, Inc. pp. 303-335. [ Links ]
5. Caviedes-Vidal E (2003). La absorción intestinal y sus implicancias ecológicas y evolutivas. In: Fisiología ecológica & evolutiva. Teoría y casos de estudio en animales. Bozinovic F. Ed. Ediciones Universidad Católica de Chile. Santiago, Chile. pp 151-168. [ Links ]
6. Cheng H, Leblond CP (1974). Origin, differentiation and renewal of the four main epithelial cell type in the mouse small intestine. I. Columnar cell. Am J Anat. 141: 461-480. [ Links ]
7. Ciminari ME, Moyano G, Chediack JG, Caviedes-Vidal E (2005). Feral pigeons in urban environments: dietary flexibility and enzymatic digestion? Revista Chilena de Historia Natural 78: 267-279. [ Links ]
8. Dahlqvist A (1984). Assay of intestinal disaccharidases. Scan J Clin Invest. 44: 69-172. [ Links ]
9. Dopido R, Rodriguez C, Gomez T, Acosta NG, Diaz M (2004). Isolation and characterization of enterocytes along the intestinal tract of the gilthead seabream (Sparus aurata L.). Comp Biochem Physiol A Mol Integr Physiol. 139(1): 21-31. [ Links ]
10. Eade OE, Andre-Ukena SS, Beeken WL (1981). Comparative Viabilities of Rat Intestinal Epithelial Cells Prepared by Mechanical, Enzymatic and Chelating Methods. Digestion 21: 25-32. [ Links ]
11. Fan MZ, Stoll B (2001). Enterocyte digestive enzyme activity along the crypt- villus and longitudinal axes in the neonatal pig small intestine. J Anim Sci. 79: 371-381. [ Links ]
12. Fan MZ, Stoll B, Jiang R, Burrin DG (2001). Enterocyte digestive enzyme activity along the crypt-villus and longitudinal axes in the neonatal pig small intestine. J Anim Sci. 79(2): 371-381. [ Links ]
13. Ferraris RP, Villenas SA, Diamond J (1992). Regulation of brushborder enzyme activities and enterocyte migration rates in mouse small intestine. Am J Physiol. 262: G1047-G1059. [ Links ]
14. Garriga C, Moretó M, Planas JM (1997). Hexose transport across the basolateral membrane of the chicken jejunum. Am J Physiol. 272: R1330-R1335. [ Links ]
15. Geyra A, Uni Z, Sklan D (2001). Enterocyte dynamics and mucosal development in the posthatch chick. Poult Sci. 80(6): 776-782 [ Links ]
16. Hartmann F, Owen R, Bissell DM (1982). Characterization of isolated epithelial cells from rat small intestine. Am J Physiol. 242(2): G147-155. [ Links ]
17. Kaeffer B (2002). Mammalian intestinal epithelial cells in primary culture: a mini-review. In vitro Cell Dev Biol Anim. 38(3): 123-134. [ Links ]
18. King DE, Asem EK, Adeola O (2000). Ontogenetic development of intestinal digestive functions in white Pekin ducks. J Nutr 130: 57-62. [ Links ]
19. Kurihara N, Paulson GD, Otto S, Miyamoto J, Hollingworth RM (1993). Use of isolated cells to study the metabolism of agrochemicals in animals. Pure & Appl Chem. 65(10): 2299-2312. [ Links ]
20. Lignot JH, Helmstetter C, Secor SM (2005). Postprandial morphological response of the intestinal epithelium of the Burmese python (Python molurus). Comp Biochem Physiol A Mol Integr Physiol. 141(3): 280-291. [ Links ]
21. Lowry OH, Rosebrough, NJ, Farr AL, Randall RJ (1951). Protein measurement with the folin phenol agent. J Biol Chem. 193: 265-275. [ Links ]
22. MacKenzie NM (1985). Comparison of the metabolic activities of enterocytes isolated from different regions of the small intestine of the neonate. Biology of the Neonate. 48: 257-268. [ Links ]
23. Martínez del Río C (1990) Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiol Zool. 63: 987-1011. [ Links ]
24. Perreault N, Beaulieu JF (1998). Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res. 245: 34-42. [ Links ]
25. Pothier P, Hugon JS (1980). Characterization of isolated villus and crypt cells from the small intestine of the adult mouse. Cell Tissue Res. 211(3): 405-418. [ Links ]
26. Quaroni A, May RJ (1980). Establishment and characterization of intestinal epithelial cell cultures. Methods in Cell Biology. Volume 21B. Academic Press, Inc. 403-427. [ Links ]
27. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ (1979). Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248-265. [ Links ]
28. Rasband WS (1997-2006). ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij [ Links ]
29. Raul F, Simon P, Kedinger M, Haffen K (1977). Intestinal enzymes activities in isolated villus and crypt cells during postnatal development of the rat. Cell Tissue Res. 12; 176(2): 167-178. [ Links ]
30. Rowling PJ, Sepulveda FV (1984). The distribution of (Na+ + K+)-ATPase along the villus crypt-axis in the rabbit small intestine. Biochim Biophys Acta. 771(1): 35-41. [ Links ]
31. Semenza G, Auricchio S, Mantei N (2001). Small-intestinal disaccharidases In: Metab Molec Bases of Inherit Disease. Scriver, Ch.R., Beaudet, A.L., Sly, W.S., and Valle, D. (Eds.), Childs, B. Kinzler, K.W, and Vogelstein, B. (Ass. Eds.), 8th Edition, Chapt. 75; McGraw-Hill, New York, pp. 1623-1650. [ Links ]
32. Smith MW (1985). Expression of digestive and absorptive function in differentiating enterocytes. Ann Rev Physiol. 47: 247-260. [ Links ]
33. Traber PG (1990). Regulation of sucrase-isomaltase gene expression along the crypt-villus axis of rat small intestine. Biochem Biophys Res Commun. 173(3): 765-773. [ Links ]
34. Traber PG (1999). Development of brush border enzyme activity. Development of the Gastrointestinal Tract. Sanderson IR and Walker WA (Eds). Hamilton, Ontario. Canada. pp 103-122. [ Links ]
35. Trypan Blue. Mediatech Technical Information. www.cellgro.com [ Links ]
36. Uni Z, Ganot S, Sklan D (1997). Posthatch development of mucosal function in the broiler small intestine. Poultry Science 77: 75-82. [ Links ]
37. Uni Z, Platin R, Sklan D (1998). Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. J Comp Physiol B. 168: 241-247. [ Links ]
38. Uni Z, Tako E, Gal-Garber, Sklan D (2003). Morphological, molecular and functional changes in the chicken small intestine of the late-term embryo. Poult Sci. 82(11): 1747-1754. [ Links ]
39. Velge P, Bottreau E, Querré P, Pardon P, Nicolle JC, Morisson M, Bout D, Dimier I (2002). Establishment and characterization of partially differentiated chicken enterocyte cell clones. Eur J Cell Biol. 81: 203-212. [ Links ]
40. Walters RJ (1993). Ion channel regulation in small intestinal crypts. Ph.D. Thesis. University of Cambridge. UK. http://www.cellscience.com/author.htm. [ Links ]
41. Weiser MM (1973). Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indication of cellular differentiation. J Biol Chem. 248: 2536-2541. [ Links ]
42. Weng XH, Beyenbach KW, Quaroni A (2005). Cultured monolayers of the dog jejunum with the structural and functional properties resembling the normal epithelium. Am J Physiol Gastrointest Liver Physiol. 288(4): G705-717. [ Links ]
43. Wolffram S, Grenacher B, Scharrer E (1998). H+-Coupled uphill transport of the dipeptide glicylsarcosine by bovine intestinal brush- border membrane vesicles. J Dairy Sci 81: 2595-2603. [ Links ]
Received on October 8, 2007.
Accepted on April 7, 2008.














