Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.33 n.2 Mendoza mayo/ago. 2009
ORIGINAL ARTICLES
Novel neurotrophic factor secreted by amniotic epithelial cells
Sankar Venkatachalam, Tamilselvi Palaniappan, Prem Kumar Jayapal, Sridharan Neelamegan, Sridhar Skylab Rajan, and Vijaya Prakash Krishnan Muthiah
Department of Anatomy, Dr. Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai 600 113, INDIA.
*Address correspondence to: Sankar Venkatachalam. Department of Anatomy, Dr. Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai 600 113, INDIA. E-mail: venkatsankar@yahoo.com / venkatachalam.sankar@gmail.com
ABSTRACT: By virtue of expressions of glial and neural surface markers and capability of neurotransmitter metabolism, amniotic epithelial cells are considered as candidate cell type for transplantation strategies to treat neurological disorders. Previously, we have reported neurotrophism exhibited by human amniotic epithelial cells when transplanted after spinal cord injury in bonnet monkeys. Amniotic epithelial cells were believed to secrete an "Epidermal Growth Factor (EGF) - like" factor and exact identification was not made. At this juncture, through the present study it was found that, chicken neural retinal cells when grown alone failed to survive and contrarily when either co-cultured with chicken amniotic epithelial cells / cultured in amniotic epithelial cell conditioned medium not only survived but also showed extensive differentiation. Fibroblast Growth Factor - 2 (FGF-2) plays a critical role in retinal development especially in chicken neural retinal development. However, immunoassay using western blot did not revealed the presence of any already known isoforms of FGF-2 in the medium. It is interesting to note that while factor secreted by amniotic epithelial cells resembles EGF and/or FGF-2 in its biological action, known isoforms of them were not detected. Considering the biological closeness between EGF and FGF-2, results indicate the possibility of a novel isoform of these growth factors secreted by amniotic epithelial cells. Further studies will establish the nature of this novel factor which will enhance the application of this interesting cell type for neural transplantations.
Key words: Neuroprotection; Neural retina development; FGF-2; Neurotrophism; Placental stem cells.
Introduction
Amniotic epithelial cells were known to express some of neuronal and glial cell markers (Sakuragawa et al., 1996) and were found capable of secreting neurotransmitters (Elwan and Sakuragawa, 1997). These findings suggested the usefulness of them as an alternate source of cells for transplantation approaches to treat neurological disorders (Sakuragawa et al., 1997).
Transplantation of amniotic epithelial cells in various regions of central nervous system such as caudate nucleus (Bankiewicz et al., 1994; Kakishita et al., 2000; 2003), hippocampus (Okawa et al., 2001) and spinal cord (Sankar and Muthusamy, 2003) had been reported. Amniotic epithelial cells transplantation in Parkinson model of rats was found to reverse the condition and prevent death in neurons (Kakishita et al., 2000; 2003). Similarly, when transplanted into ischemic cortical areas, they were found to differentiate into "neuron-like" cells (Okawa et al., 2001). In our previous report, we concluded the usefulness of amniotic epithelial cells transplantation in spinal cord injury repair research. We had also outlined various biological and social advantages by which, amniotic epithelial cells transplantation excels its precedent viz. neural transplantation (Sankar and Muthusamy, 2003). Recently, our conclusion of usefulness of amniotic epithelial cells transplantation to treat spinal cord injuries has been confirmed in rodent models of spinal cord injury (Zhi-yuan et al., 2006; our unpublished observations).
It is imperative to understand the mechanism by which these cells exhibit neurotropism. Based on the neurotropism exerted by amniotic epithelial cells conditioned medium, a diffusible neurotrophic factor produced by them had been suggested as a possible cause apart from direct cell-to-cell effects (Uchida et al., 2003). Attempts to identify the neurotrophic factor were not successful. Sakuragawa et al. (2001) had found amniotic epithelial cells conditioned medium showed neurotrophic effect on rat embryonic day 18 (E18) cortical neurons. As E18 cortical neurons would not normally respond to neurotrophic factors other than Epidermal Growth Factor (EGF), they hypothesized that it could be EGF. However, as they could not prove the presence of EGF in the conditioned medium by assay methods, they have concluded it as "novel neurotrophic factor" and it could be "EGF-like". Nevertheless, the exact mechanism is yet to be identified.
At this juncture, in continuation of our previous work on amniotic epithelial cells, we attempted to find out the possible mechanism behind the neurotropism exhibited by these cells. Results obtained from our present work indicate that amniotic epithelial cells might be secreting a "novel" neurotrophic factor which could be "Fibroblast Growth Factor (FGF) -like".
Material and Methods
Protocol of this study was approved by Institutional Animal Ethical Committee (IAEC) of Dr.Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai 600 113.
Cells and their obtainment
For experiments, amniotic epithelial cells from both humans and chicken, as well as chicken neural retinal cells were used. Human placenta obtained after cesarean delivery were washed several times in ringer lactate solution and amniotic membrane was peeled off from the chorionic surface. Connective tissue adherent to the membrane was thoroughly removed by scrubbing and was cut into pieces. Fertilized white leghorn eggs were incubated and embryos of 10 days of age were removed as per standard procedures (Freshney, 2000). Amnion surrounding the embryos was carefully removed and kept separately. Extreme care was excised not to include blood vessels and other fetal membranes along with the isolated amnion. Neural retinal layer was peeled off from its surrounding pigment epithelial layer and collected separately. Isolated human amnion, chicken amnion and chicken neural retina were incubated in 0.175% trypsin in Hank's solution at 37ºC for 30 minutes. Cells obtained were dispersed by gentle trituration, washed in Hank's solution for 3 times and collected in medium; composition of which is given below.
Culture conditions
Cells were seeded on poly-L-lysin coated cover slips in multi well plates. Culture medium comprised of Dulbeco's Minimum Essential Medium (DMEM) + F12 at 1:1 ratio supplemented with 10 mM L-Glutamine, 50 μg gentamicin/ml, 2.5 μg amphotericin B/ml and 10% fetal bovine serum. Culture plates were incubated in a humidified atmosphere with 5% CO2 at 37ºC. Cultures were interrupted after 24 hours and 48 hours of incubation. No in-between medium change was done during the maximum observation period of 48 hours.
Preparation of conditioned medium
Two types of conditioned medium were prepared; one by growing chicken amniotic epithelial cells and another by growing human amniotic epithelial cells. After growing cells for 24 hours in the above mentioned culture conditions, the medium was collected, centrifuged at low speed (1000 rpm for 5 minutes) to remove debris and used immediately after filtered through 0.2 micron filter. Medium used for cultures comprised of 50% conditioned medium and 50% fresh medium.
Cell labeling
In co-culture experiments as given below, chicken amniotic epithelial cells were labeled with CellTracker Green BODIPY (Invitrogen, USA) and chicken neural retinal cells were labeled with 1, 1' - dioctadecyl - 3, 3, 3', 3' - tetramethyllindocarbocyanine perchlorate (DiI) (Molecular Probes, USA). To label chicken amniotic epithelial cells, they were suspended in Hank's solution containing 20 micromoles of CellTracker Green dye and incubated for 30 minutes at 37ºC. Cells were then transferred to fresh medium and incubated for 30 minutes at 37ºC in 5% CO2 in air. After this, cells were washed once and re-suspended in fresh medium to the final required concentration for seeding. To label chicken neural retinal cells, they were suspended in Hank's solution with 10 micromoles of DiI (Molecular Probes, USA) and were incubated for 5 minutes at 37ºC followed by 15 minutes at 4ºC. After this, cells were washed once and re-suspended in fresh medium to the final required concentration for seeding.
Culture in conditioned medium and co-cultures
The following combinations were performed using cells seeded on poly-L-Lysine coated cover slips kept in 12 - well tissue culture plates.
Group 1 - Chicken amniotic epithelial cells
Group 2 - Chicken neural retinal cells
Group 3 - Chicken neural retinal cells + chicken amniotic epithelial cells
Group 4 - Chicken neural retinal cells + chicken amniotic epithelial cells conditioned medium
Group 5 - Chicken neural retinal cells + human amniotic epithelial cells conditioned medium
Seeding was carried out in such a way that each well would contain 1 x 106 cells. In combination group i.e. Group 3; both the types of cells were seeded in 1:1 proportion with a final concentration of 1 x 106 cells in the well. Each well contained 2 ml of culture medium.
Immunofluorescence and fluorescence microscopy
Cover slips with unlabelled cells were fixed in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) at pH 7.2. Then they were blocked in 10% normal goat serum in 0.3% Triton-X 100 in PBS and were incubated with rabbit anti-NF 200 (1:200 dilution) or rabbit anti-GFAP (1:200 dilution) diluted with 3% normal goat serum in 0.3 Triton-X 100 in PBS. Primary antibodies were visualized using fluorescein isothiocyanate conjugated goat anti-rabbit IgG (1:200 dilution with 3% normal goat serum in 0.3% Triton-X 100 in PBS). Cells in co-culture experiments were fixed in the above manner and mounted in glycerine jelly for visualization. For fluorescence viewing, upright epi-fluorescence microscope with either fluorescein isothiocyanate or Rhodamine filter sets were used for fluorescein isothiocyanate and CellTracker Green or DiI respectively.
Light microscopy and histology
In separate set of cover slips, viability of the cells was estimated using 0.1% trypan blue (Freshney, 2000) and were subsequently stained with 1% toluidine blue (Culling, 1972). Eyeballs of chick embryos of 10th, 11th and 12th day of incubation were fixed in 10% formal saline and subjected to paraffin processing. Sections were cut at 7 μ thickness and were stained with 1% toluidine blue or Glees and Marshland modification of Belchowsky's method (Culling, 1972).
Western Blot
Cells were allowed to grow for 24 hours before being harvesting medium for analysis. Protease inhibiting cocktails were added to conditioned medium and chicken and human amniotic epithelial cell lysates prepared with cell lysis buffer. Protein concentrations were determined by the method of Lowry et al. (1951). Equal amounts of protein (50 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transblotted onto PVDF membranes.
Membranes, blocked with 10% nonfat dry milk in 50 mM Tris-buffered saline (pH 8.0) with 0.2% tween-20 (TBS-T) at room temperature for 3 hrs, were incubated with primary antibody diluted in TBS-T: antibFGF rabbit polyclonal antibody (1:500 dilution; Merck biosciences) for overnight at 4ºC. After incubated with secondary anti rabbit IgG conjugated to horse radish peroxidase at 1:5000 dilution for one hour at room temperature, antibody binding was visualized using luminol reagent and X-ray film (Pierce). Band intensity was measured by multianalyst (Bio-Rad); the values are expressed in arbitrary units. In some cases, gels containing duplicate samples were stained with ponceau red to visualize all bands. Control blots were performed with homogenates from rat fetal liver, fetal kidney and postnatal 1 month old pup kidney tissues.
Results
After 24-48 hrs of culture, chicken amniotic epithelial cells were seen surviving without any obvious cell loss and there was no change in their morphology (Fig. 1A). On the other hand, chicken neural retinal cells failed to survive well and there was extensive cell death. Few cells seen surviving were without any fiber outgrowth and without any change in morphology (Fig. 1B).
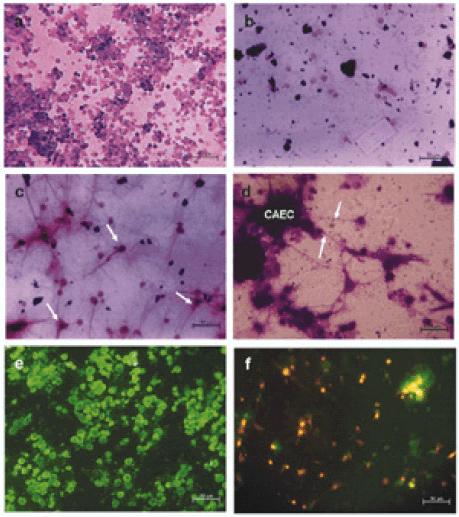
FIGURE 1. Cell culture studies. a. Chicken amniotic epithelial cells cultured for 24 hours. Morphology of cells did not change. Some dividing cells were seen. There was no significant cell death. b. Chicken neural retinal cells cultured for 24 hours. There was significant cell loss. Few surviving cells were seen without any neurite outgrowths / morphology changes. c. Chicken neural retinal cells cultured with chicken amniotic epithelial cells conditioned medium after 24 hours. Survival of chicken neural retinal cells in such conditioned medium was high. Almost all surviving cells show neurite outgrowths. Morphology was characteristic of neurons. Arrows indicate some of the cells with typical neuronal morphology. See also Fig. 1E. d. Chicken neural retinal cells co-cultured with chicken amniotic epithelial cells. Amniotic epithelial cells (CAEC) formed aggregates and neurites of neural retinal cells were seen growing towards them (arrows), indicating the trophic influence of amniotic epithelial cells over the processes of neural retinal cells. e. Anti-NF- 200 staining of neural retinal cells cultured in the presence of amniotic epithelial cells conditioned medium. All the surviving cells were positive for neurofilament-200 (NF-200) and negative for glial fibrillary acidic protein (GFAP) (data not shown). f. Fluorescent image of co-cultures of DiI labeled neural retinal cells and CellTracker Green labeled amniotic epithelial cells. Green fluorescent amniotic epithelial cells can be seen aggregated and DiI positive neural retinal cells can be seen with neurite outgrowths. At many places, neural retinal cells were seen in close association with amniotic epithelial cell aggregates. Scale bars in photomicrographs represent 50 microns.
In chicken amniotic epithelial cells conditioned medium cultures, chicken neural retinal cells were found to have differentiated (Fig. 1C). Similar effects were seen with human amniotic epithelial cells conditioned medium (Data not shown). Immunofluorescence studies showed that such differentiated retinal cells were positively stained for neurofilament -200 (NF 200) (Fig. 1E) and were negative for glial fibrillary acidic protein (GFAP).
In co-cultures, chicken amniotic epithelial cells remained as undifferentiated cells. However, chicken neural retinal cells were found not only surviving well but also showed differentiation. Typical multipolar neurons were seen and frequently their neurites were seen grown towards and attached to chicken amniotic epithelial cell aggregates (Fig. 1D). Co-culture of these cells with different fluorescent labels confirmed this observation (Fig. 1F).
Eyeballs processed for histology showed normal features such as bi-layered retina and other coverings along with optic nerve. The cells of retina were seen as undifferentiated round cells without any evidence of neurite growth (Fig. 2A and 2B). The results were scored in a semiquantitative manner by observers who were unaware of the experiment protocol and the scores were given as Table 1.
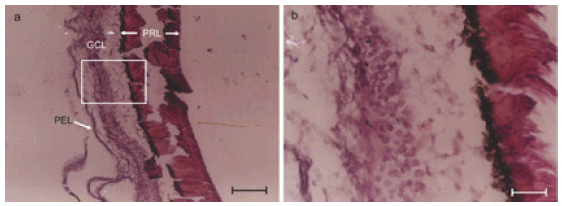
FIGURE 2. Eye ball histology showing undifferentiated cells at 11 days. a. Eye ball histology of 11 day old chick embryo. Histochemical staining for axons using Glees and Marshland modification of Bielchowsky's Method. Various layers of the retina viz. photoreceptor layer (PRL), ganglion cell layer (GCL) and pigment epithelial layer (PEL) can be seen. Boxed area is shown enlarged in Fig. 2B. b. Magnified view of boxed area in Fig. 2A. Cells in the ganglion cell layer were seen without any processes / neurites on 11th day of development in chick embryo. Compare this with extensive neurite development by 10th day chicken neural retinal cells in culture after 24 hours (comparable to 11th embryonic day) in the presence of factors present in chicken amniotic epithelial cells conditioned medium.
TABLE 1. Semi-quantitative assessment of survival and/or differentiation of cells under different experimental conditions.
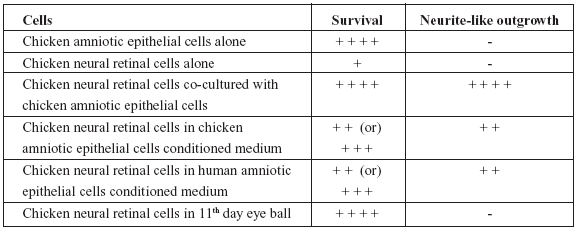
From the Table 1, it would be obvious that while chicken amniotic epithelial cells were unaffected, neural retinal cells when cultured along with chicken amniotic epithelial cells or in conditioned medium obtained from either chicken or human amniotic epithelial cells not only survived but also showed differentiation. Although, neural retinal cells in vivo survived, there was no differentiation by 11th day of incubation (24 hours past 10th day of incubation).
Western blot indicated FGF-2 positive bands in positive controls which correspond to the four known isoforms (with a molecular weight of 18, 18.5, 22 and 22.5 kDa) of FGF-2. No FGF-2 was detected in conditioned mediums or in cell lysates (Figs. 3A and 3B), indicating the absence of the already known four isoforms of FGF-2 in both conditioned mediums and cell lysates. Also, neither the serum used for cultures or control culture medium contained detectable levels of FGF-2 in the western blot.

FIGURE 3. Western blot of chicken amniotic epithelial cells conditioned medium, chicken amniotic epithelial cells lysate, human amniotic epithelial cells conditioned medium, human amniotic epithelial cells lysate did not revealed presence of FGF-2. Fetal rat liver, kidney or postnatal rat pup kidney homogenates were used as positive controls. Blots indicate the FGF-2 antibody used was able to detect all the known isoforms of FGF-2 viz. 18, 18.5, 22 and 22.5 kDa variants. Absence of bands in the test samples indicates absence of known isoforms of FGF-2 in them.
Discussion
The mechanism behind the neurotrophism exhibited by amniotic epithelial cells appears to be complex. As Sakuragawa et al. (2001) and Uchida et al. (2000; 2003) opined EGF-like factor may be responsible, involvement of a factor closely related to EGF was explored. Possibility of FGF-2 secretion by amniotic epithelial cells was envisaged based on an incidental finding of overgrowth of fibroblasts in the presence of these cells than when alone in cultures.
Chicken neural retinal cells were selected for testing the bio-activity of the product secreted by amniotic epithelial cells. Chicken neural retina development heavily depends on FGF-2 (Cirillo et al., 1990; Pittack et al., 1997; Desire et al., 1998). Blocking the action of endogenous FGF-2 caused malformation of nervous structures (Dono et al., 1998; Szebenyi et al., 2001; Raballo et al., 2000) especially retina (McFarlane et al., 1998; Martinez-Morales et al., 2005). Action of FGF-2 on chicken retinal cells with neural potentials appears to be dose dependent. For e.g. in developing eye ball, endogenous FGF-2 cause pigment epithelium to divide while excess FGF-2 from exogenous source would lead to the differentiation of pigment epithelium towards neural line (Azuma et al., 2005) and double neural retina would arise out of such situation (Tcheng et al., 1994). Thus, in low concentrations, FGF-2 induces mitosis in the neural retinal cells to increase the cell population while in high concentrations, cause differentiation in the cells. These previous studies indicate that neural retinal cells from developing eyeball could be used for bio-assaying FGF-2 qualitatively if not quantitatively. Practically, usage of chicken retinal and amniotic cells rather than rat cells gave an advantage in terms of ethical aspects as chicken cells can be obtained without sacrificing a pregnant animal.
The results of the present study indicates that the neurotrophic factor secreted by amniotic epithelial cells mimics FGF-2 in action on the developing chicken neural retinal cells. Why amniotic epithelial cells should secrete FGF-2? Amniotic epithelial cells require FGF-2 for their maintenance i.e. division and survival (Gospodarowicz et al, 1977; Chettur et al., 1978; Porreco et al., 1980). FGF-2 secreted by amniotic epithelial cells in an autocrine basis might find its way into the amniotic fluid and might influence the development of nervous system. A diffusible neurotrophic factor from amnion is thought to induce the earliest neural development i.e. neural tube development from the totipotent epiblast cells (Streit et al., 2000) at which stage nothing is intervening between amnion and epiblast cells except the amniotic cavity with fluid. Thus in the early stages of development, amniotic epithelial cells might function as exogenous FGF-2 suppliers supplementing endogenous sources for the development of cells.
When induction of neural tube and neural derivatives such as optic cup were initiated, gradually the developing neural structures (such as retina) get away from surface and FGF-2 secreted by amnion may no longer diffuse through great distances to reach those structures.
Such protection from the excess exogenous FGF-2 may be necessary to avoid pre-mature differentiation resulting in less cell population. Interestingly, in the present study also, eyeball of chick embryo on 11th and 12th day did not showed differentiated cells in retina indicating that they are still capable of increasing their population. However, the same cells of comparable age (10th day cells maintained for 24 to 48 hours days in culture) when cultured along with amniotic epithelial cells or with their conditioned medium showed extensive differentiation. It may be interpreted as in vivo, the cells of neural retina were kept from differentiation by the controlled (limited) supply of endogenous FGF-2. When they are exposed to excess amount of FGF-2 in vitro, secreted by amniotic epithelial cells, differentiation might have been induced. These observations indicate that in the given concentrations and ratio, chicken amniotic epithelial cells can secrete a factor which is capable of inducing differentiation in chicken neural retinal cells and this factor could be FGF-2 like in action due to reasons outlined earlier.
However, in western blot no already established isoforms of FGF-2 was detected although the antibody used in the present study was capable of detecting all the known isoforms of FGF-2. Koizumi et al. (2000) had showed human amniotic epithelial cells exhibit both gene and protein expression for both FGF and EGF along with several other factors. Nevertheless, based on biological action neither Sakuragawa (2001) who concluded "EGF like" nor we who concluded "FGF-2 like" could confirm the presence of known isoforms of EGF and FGF-2 respectively through immunoassays. Koizumi et al. (2000), used cryopreserved amniotic membranes for their study. Sakuragawa et al. (2001) and we for the current study used primary cultures of amniotic epithelial cells from freshly obtained placenta and also the culture conditions were different. It is not know at present whether these variations are responsible for the observed differences.
Neuroprotective effects of human amniotic epithelial cells secreted factors were found to be higher than those of BDNF, NT-3, CNTF or their combinations and therefore, these cells were considered to produce unknown neurotrophic factor other than those three factors (Uchida et al., 2000; Sakuragawa et al., 2001; Uchida et al., 2003). Given that already possibility of BDNF, NT-3, CNTF and EGF mediated mechanism behind neurotropism exhibited by amniotic epithelial cells was ruled out, observations of the present study adds FGF-2 to that list.
Interestingly, both EGF and FGF-2 are related to each other in several aspects. Both are mesenchymal mitogens. Both EGF and FGF exhibit similar actions on developing central nervous system as well as adult neural stem cells (Goh et al., 2003). Exposure to FGF-2 is a pre-requisite for the development of EGF responsiveness in developing neural cells (Ciccolini and Svendsen, 1998). They have a common antagonist viz. "SPRY" (Kramer et al., 1999). Both are capable of rescuing injured neural cells (Traverso et al., 2003). During development, neural stem cells such as sub-ventricular zone cells respond to FGF-2 and EGF by co-expressing receptors for both (Gritti et al., 1999; Kalyani et al., 1999). Finally, in the mRNA sequences, EGF and FGF-2 showed a homology score of 9446 (in European Molecular Biology Laboratory (EMBL), European Bioinformatics Institute (EBI) database search), which is a closer relation than most other neurotrophic factors as given in Tables 2 and 3.
TABLE 2. Homology score of EGF mRNA with other neurotrophic factors (Higher the score indicates more closer similarity in the sequences)
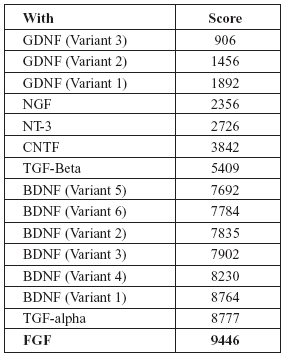
TABLE 3. Homology score of FGF-2 mRNA with other neurotrophic factors (Higher the score indicates more closer amino acid sequence similarity)

From the foregoing discussions, it may be speculated that the neurotrophic factor secreted by amniotic epithelial cells might be closely related to EGF and/or FGF-2. In this aspect it may be an unidentified isoform of any of these two factors. Contrarily, amniotic epithelial cells may be capable of secreting an array of neurotrophic / growth factors in a spatio-temporal manner as the culture conditions of the studies were different from each other. This dynamic possibility of amniotic epithelial cells secreting different factors cannot be negated as Koizumi et al. (2000) showed both gene and protein expression of several factors by amniotic epithelial cells.
Neurotrophic factor secreted by amniotic epithelial cells does not appear to be species specific as human amniotic epithelial cells secreted factor acts on both rat (Uchida et al., 2003) and chick neural cells [present study]. Therefore, an approach using chick embryo derived neural and amniotic epithelial cells along with human amniotic epithelial cells as used in our study could be a suitable model for want of ethical reasons. Because, procurement of human amniotic epithelial cells does not impose much ethical restrictions and getting cells from chick embryo does not necessitate the sacrifice of a pregnant animal to get developing neural cells. Moreover, using (chick) embryos of half-of the gestation age may not require ethical clearance in certain countries (Freshney, 2000).
Amniotic epithelial cells were found to express characteristic features of stem cells (Miki et al., 2005) and have high potential for being used in transplantations (Bailo et al., 2004) as a stem cell. Since amniotic epithelial cells were found to be not only immuno logically naive but also secrete immunosuppressive factors (Li et al., 2005), they may not evoke immune rejection problems thus avoiding the immunosupression and its complications. In this aspect, they can be considered advantageous when compared with other mesenchymal stem cells. However, mechanism behind amniotic epithelial cell mediated neurotropism needs to be identified in order to carry forward this research towards clinical applications. Further research is warranted in this direction to explore about this interesting cell type which appears to be highly dynamic in its functions.
Acknowledgements
Study supported by research grants from University Grants Commission, Government of India (Grant Reference Number: UWPFEP - University of Madras - HS22) and Research Starter Fund from University of Madras. Presented (Oral presentation) in First International Workshop on Placenta Derived Stem Cells held in Brescia, Italy March 23-24, 2007.
References
1. Azuma N, Tadokoro K, Asaka A (2005). Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Human Molecular Genetics 14: 1059-1068. [ Links ]
2. Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, Albertini A, Zorzi F, Cavagnini A, Candotti F, Wengler GS, Parolini O (2004). Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78: 1439-1448. [ Links ]
3. Bankiewicz KS, Palmatier M, Plunkett RJ, Cummins A, Oldfield EH (1994). Reversal of hemiparkinsonian syndrome in nonhuman primates by amnion implantation into caudate nucleus. Journal of Neurosurgery 81: 869-876. [ Links ]
4. Chettur L, Christensen E, Philip J (1978). Stimulation of amniotic fluid cells by fibroblast growth factor. Clinical Genetics 14: 223-228. [ Links ]
5. Ciccolini F, Svendsen CN (1998). Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. Journal of Neuroscience 18: 7869-7880. [ Links ]
6. Cirillo A, Arruti C, Courtois Y, Jeanny JC (1990). Localization of basic fibroblast growth factor binding sites in the chick embryonic neural retina. Differentiation 45: 161-167. [ Links ]
7. Culling CFA (1972). Handbook of histopathological and histochemical techniques. 3rd Edition. London: Butterworths. [ Links ]
8. Dono R, Texido G, Dussel R, Ehmke H, Zeller R (1998). Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. The EMBO Journal 17: 4213-4225. [ Links ]
9. Desire L, Head MW, Fayein NA, Courtois Y, Jeanny JC (1998). Suppression of fibroblast growth factor-2 expression by antisense oligonucleotides inhibits embryonic chick neural retina cell differentiation and survival in vivo. Developmental Dynamics 212: 63-74. [ Links ]
10. Elwan MA, Sakuragawa N (1997). Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. NeuroReport 8: 3435-3438. [ Links ]
11. Freshney RL (2000). Culture of animal cells: a manual of basic techniques. 1st Edition. New York: Wiley-Liss. [ Links ]
12. Goh ELK, Ma D, Ming GL, Song H (2003). Adult neural stem cells ad repair of adult central nervous system. Journal of Hematotherapy & Stem Cell Research 12: 671-679. [ Links ]
13. Gospodarowicz D, Moran JS, Owashi ND (1977). Effects of fibroblast growth factor and epidermal growth factor on the rate of growth of amniotic fluid-derived cells. Journal of Clinical Endocrinology and Metabolism 44: 651-659. [ Links ]
14. Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL (1999). Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. Journal of Neuroscience 19: 3287-3297. [ Links ]
15. Kakishita K, Elwan MA, Nakao N, Itakura T, Sakuragawa N (2000). Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson's disease: A potential source of donor for transplantation therapy. Experimental Neurology 165: 27-34. [ Links ]
16. Kakishita K, Nakao N, Sakuragawa N, Itakura T (2003). Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6- hydroxydopamine lesions. Brain Research 980: 48-56. [ Links ]
17. Kalyani AJ, Mujtaba T, Rao MS (1999). Expression of EGF receptor and FGF receptor isoforms during neuroepithelial stem cell differentiation. Journal of Neurobiology 38: 207-224. [ Links ]
18. Koizumi N, Inatomi T, Sotozono C, Fullwood NJ, Quantock AJ, Kinoshita S (2000). Growth factor mRNA and protein in preserved human amniotic membrane. Current Eye Research 20: 173-177. [ Links ]
19. Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y (1999). Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development 126: 2515-2525. [ Links ]
20. Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H (2005). Immunosuppressive Factors Secreted by human amniotic epithelial cells. Investigations in Ophthalmology and Vision Science. 46: 900-907. [ Links ]
21. Lowry OH, Rosbrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265-275. [ Links ]
22. Martinez-Morales JR, Bene FD, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J (2005). Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Developmental Cell 8: 565-574. [ Links ]
23. McFarlane S, Zuber ME, Holt CE (1998). A role for the fibroblast growth factor receptor in cell fate decisions in the developing vertebrate retina. Development 125: 3967-3975. [ Links ]
24. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005). Stem cell characteristics of amniotic epithelial cells. Stem Cells 23: 1549-1559. [ Links ]
25. Okawa H, Okuda O, Arai H, Sakuragawa N, Sato K (2001). Amniotic epithelial cells transform into neuron-like cells in the ischemic brain. Neuroreport 12: 4003-4007. [ Links ]
26. Pittack C, Grunwald GB, Reh TA (1997). Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 124: 805-816. [ Links ]
27. Porreco RP, Bradshaw C, Sarkar S, Jones OW (1980). Enhanced growth of amniotic fluid cells in presence of fibroblast growth factor. Obstetics and Gynecology 55: 55-59. [ Links ]
28. Raballo R, Rhee J, Cook RL, Leckman JF, Schwartz ML, Vaccarino FM (2000). Basic fibroblast growth factor (FGF-2) is necessary for cell proliferation and neurogenesis in developing cerebral cortex. Journal of Neuroscience 20: 5012-5023. [ Links ]
29. Sakuragawa N, Thangavel R, Mizuguchi M, Hirasawa M, Kamo I (1996). Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neuroscience Letters 209: 9-12. [ Links ]
30. Sakuragawa N, Misawa H, Ohsugi K, Kakishita K, Ishii T, Thangavel R, Tohyama J, Elwan M, Yokoyama Y, Okuda O, Arai H, Ogino I, Sato K (1997). Evidence for active acetylcholine metabolism in human amniotic epithelial cells: applicable to intracerebral allografting for neurologic disease. Neuroscience Letters 232: 53-56. [ Links ]
31. Sakuragawa N, Elwan MA, Uchida S, Fujii T, Kawashima K (2001). Non-neuronal neurotransmitters and neurotrophic factors in amniotic epithelial cells: expression and function in humans and monkey. Japanese Journal of Pharmacology 85: 20-23. [ Links ]
32. Sankar V, Muthusamy R (2003). Role of amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 118: 11-17. [ Links ]
33. Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD (2000). Initiation of neural induction by FGF signaling before gastrulation. Nature 406: 74-78. [ Links ]
34. Szebenyi G, Dent EW, Callaway JL, Seys C, Lueth H, Kalil K. (2001). Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. Journal of Neuroscience 21: 3932-3941. [ Links ]
35. Tcheng M, Oliver L, Courtois Y, Jeanny JC (1994). Effects of exogenous FGFs on growth, differentiation, and survival of chick neural retina cells. Experimental Cell Research 212: 30-35. [ Links ]
36. Traverso V, Kinkl N, Grimm L, Sahel J, Hicks D (2003). Basic fibroblast and epidermal growth factors Stimulate survival in adult porcine photoreceptor cell cultures. Investigations in Ophthalmology and Vision Science 44: 4550-4558. [ Links ]
37. Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N (2000). Neurotrophic function of conditioned medium from human amniotic epithelial cells. Journal of Neuroscience Research 62: 585-590. [ Links ]
38. Uchida S, Suzuki Y, Araie M, Kashiwagi K, Otori Y, Sakuragawa N (2003). Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells. Neuroscience Letters 341: 1-4. [ Links ]
39. Zhi-yuan W, Guo-zhen H, Yi L, Xin W, Li-he G (2006). Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chinese Medical Journal 119: 2101-2107. [ Links ]
Received: September 5, 2008.
Revised version received: April 16, 2009.
Accepted: April 19, 2009.














