Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.33 n.2 Mendoza mayo/ago. 2009
ORIGINAL ARTICLES
Impact of pituitary FSH purification on in vitro early folliculogenesis in goats
D.M. Magalhães1*, V.R. Araújo1, I.B. Lima-Verde1, M.H.T. Matos1, R.C. Silva2, C.M. Lucci2, S.N. Báo2, C.C. Campello1, and J.R. Figueiredo1
1. Faculdade de Medicina Veterinária, LAMOFOPA, PPGCV, Universidade Estadual do Ceará, Fortaleza, CE, Brasil.
2. Laboratório de Microscopia Eletrônica, Departamento de Biologia Celular, Universidade de Brasília, Brasilia, DF, Brasil
*Address correspondence to: D.M. Magalhães. Faculdade de Medicina Veterinária, LAMOFOPA, PPGCV, Universidade Estadual do Ceará, Fortaleza, CE, BRASIL. E-mail: dmmvet@hotmail.com
ABSTRACT: Porcine pituitary follicle stimulating hormone (pFSH) is known to regulate the production of growth factors that have an essential role in early foliculogenesis. However, the effects of different preparations of pFSH on the survival and development of caprine follicles are not yet known. The aim of this study was to evaluate the effects of different pFSH (Stimufol® and Folltropin®) on the in vitro survival and growth of caprine preantral follicles. Pieces of caprine ovarian tissues were cultured for either one or seven days in a supplemented Minimum Essential Medium, alone or containing either Stimufol® (50 ng/mL) or Folltropin® (10, 50, 100 and 1000 ng/mL). Fresh control ovarian tissues as well as cultured tissued were processed for histological and ultrastructural studies. The results showed that after seven days, only Stimufol® maintained follicular morphology similar to control. Moreover, follicular degeneration was higher in medium alone or with Folltropin® at 50, 100 and 1000 ng/mL. However, at day seven, the percentage of growing follicles was higher in 100 ng/mL of Folltropin® than Stimufol®. In conclusion, FSH preparations affect differently the performance of in vitro culture of caprine preantral follicles. Stimufol® was better to preserve follicular morphology while Folltropin® was more efficient to promote follicular growth.
Key words: Goat; Preantral follicles; FSH; Follicular growth
Introduction
Over recent years, several researches have been done on the factors regulating early folliculogenesis, particularly in monovular species, such as domestic ruminants (Matos et al., 2007; Saraiva et al., 2008). These researches have raised the possibility that the store of oocytes enclosed in primordial follicles could be exploited to increase the reproductive potential of economically important animals, to preserve endangered species, as well as to treat infertility in clinical medimedicine.
In addition, the in vitro culture of preantral follicles allows the evaluation of the effects of different substances (hormones, growth factors, antibiotics, etc.) before their in vivo use, either in animals or humans. Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein synthesized and secreted by the anterior pituitary gland under hypothalamic control, and which is involved in early folliculogenesis. FSH receptors are expressed in granulosa cells (Ulloa-Aguirre et al., 2003; O'Shaughnessy et al., 1996) from the primary follicle stage onward (Oktay et al., 1997). It is well known that FSH can indirectly act in the primordial follicles through paracrine factors secreted by follicular or stromal cells, such as KIT ligand (Fiona et al., 2005; Eppig, 2001). Some in vitro studies demonstrated that the addition of FSH to the culture medium promotes the inhibition of apoptosis and antrum formation in large isolated secondary follicles from different species (murine: McGee et al., 1997; human: Wright et al., 1999; ovine: Cecconi et al., 1999; bovine: Gutierrez et al., 2000; swine: Mao et al., 2002). In spite of the beneficial effects of FSH on the in vitro follicular development, Nuttinck et al. (1996) showed that pFSH (0.43 pg FSH/pg proteins) induces degeneration in small bovine preantral follicles. The differences among these studies may, in part, due to the degree of purity of commercial preparations of FSH.
FSH can be obtained by pituitary extract of domestic animals, mainly swine and sheep, followed by hormone purification (Calder et al., 2003). Even after its purification, porcine FSH (pFSH) has some contamination with other pituitary hormones, such as luteinizing hormone (LH) and thyroid stimulating hormone (TSH) (Closset and Hennen, 1989). There are two well known commercial preparations of pFSH, i.e.; Folltropin® (Bényei and Barros, 1999) and Stimufol® (Mazouz et al., 1996). The FSH:LH ratio in Stimufol® is 20:1 (Mazouz et al., 1996) and it is approximately 5.25:1 in Folltropin® (manufacturer's specifications, 2008). Both Folltropin® and Stimufol® have shown satisfactory results in the superovulation and embryo transfer protocols in different species (ovine: Cognie, 1999; bovine: Bényei and Barros, 1999; caprine: Baldassarre and Karatzas, 2004; Bonnet-Garnier, 2008). However, the effects of different commercial preparations of FSH on the survival, as well as on the in vitro development of caprine preantral follicles have not been studied.
In the current study we wanted to compare the effects of Stimufol® and Folltropin® on the viability (through both structural and ultrastructural analyses), activation of primordial follicles and further follicular growth on cultured caprine ovarian tissue.
Materials and Methods
Source and preparation of ovarian tissue
Ovaries (n = 8) from 4 adult (1 - 3 years old), mixedbred goats (Capra hircus) were obtained at a local slaughterhouse. Immediately after slaughter, the ovaries were removed, washed once in 70% alcohol and twice in Eagle's minimum essential medium (MEM) supplemented with 100 μg/mL penicillin and 100 μg/mL streptomycin. The material was transported in thermo flasks at 4ºC to the laboratory, and were submitted within 1 hour to the experimental procedures (as described below).
Caprine ovarian tissue culture
At the laboratory, both ovaries from each animal were stripped of surrounding fat tissue and ligaments and cut in half. Then the ovarian cortex was isolated by removing the ovarian medulla, large antral follicles and corpora lutea. Afterwards, the ovarian cortex was divided into 21 fragments of approximately 3 ∞ 3 mm (1 mm thick). One fragment (non-cultured control) was immediately fixed in Carnoy's fluid for 12 h for histological studies, while a smaller fragment (1 mm3) was fixed in paraformaldehyde 2% and glutaraldehyde 2.5% in sodium cacodylate buffer 0.1 M (pH 7.2) for ultrastructural examination. The other fragments of ovarian cortices were individually in vitro cultured in 1 mL of culture medium (see below) for either one or seven days at 39ºC with 5% CO2 in air using a 24-well culture dish. The culture medium used was a supplemented MEM (with insulin 6.25 μg/mL, transferrin 6.25 μg/mL and selenium 6.25 ng/mL, 0.23 mM pyruvate; 2 mM glutamine; 2 mM hypoxanthine and 1.25 mg/mL bovine serum albumin). The control medium was tested alone (cultured control) or after adding either 50 ng/mL Stimufol® (provided by Dr J.F. Beckers, Liège, Belgium) or Folltropin (Tecnopec, Brasil) in different concentrations (10, 50, 100 or 1000 ng/mL). All chemicals used in the present study were purchased from Sigma Chemical Co. unless otherwise indicated. Every 2 days, the culture medium was replaced by fresh medium and each treatment was repeated four times. The concentration of 50 ng/ml of Stimufol® was chosen because it showed better results when compared to the other concentrations (1, 10 and 100 ng/ml) in previous experiments performed in our laboratory (Matos et al., 2007).
Histological examination and assessment of in vitro follicle growth
To evaluate the morphology of caprine follicles after one or seven days of culture, the fixed tissue fragments were dehydrated in a graded ethanol series, clarified with xylene and embedded in paraffin wax. Seven ìm sections were mounted on slides, stained with periodic acid Schiff and hematoxylin, and examined by light microscopy at 100∞ and 400∞ magnification.
Follicles were classified as described by Hulshof et al. (1994) into primordial (one layer of flattened granulosa cells around the oocyte) and growing follicles. The latter were classified as "primary" (a single layer of cuboidal granulosa cells around the oocyte) or "secondary" follicles (oocyte surrounded by two or more layers of cuboidal granulosa cells). Non viable follicles were defined as those in which the oocyte was shrunk, had a pyknotic nucleus and/or was surrounded by disorganized granulosa cells (detached from the basement membrane). To evaluate follicular activation and growth, only intact follicles with a visible oocyte nucleus were recorded and the proportion of primordial and growing follicles were calculated at day 0 (control) and after one or seven days of culture in the various media tested. To avoid counting a follicle more than once, preantral follicles were counted only when the oocyte nucleus was visible. Oocyte and follicle diameters before and after culture were measured with the aid of an ocular micrometer. At least 120 follicles were randomly evaluated in each case.
Ultrastructural analyses
After fixation, the fragments were washed with sodium cacodylate buffer and postfixed in 1% osmium tetroxide, 0.8% potassium ferricyanide and 5 mM CaCl2 in 0.1 M sodium cacodylate buffer. Subsequently, samples were contrasted with uranyl acetate, dehydrated in a graded series of acetone and embedded in Spurr's epoxy resin. Follicles which were classified as histologically viable in toluidin blue-stained semi-thin sections (3 μm) were submitted to ultrastructural analysis. For that purpose, ultra-thin sections (70 nm) were cut on an ultramicrotome (Reichert Supernova, German) and examined using a Jeol 1011 (Jeol, Tokyo, Japan) transmission electron microscope, operating at 80 kV.
Statistical analyses
Kolmogorov-Smirnov and Bartlett's methods were applied to test normal distribution and homogeneity of variance, respectively. Analysis of variance was made using General Linear Models (GLM) procedure of Statistical Analysis System (SAS, 1999) and Dunnett's test was applied for comparison of control groups against each treatment tested. Student Newman Keuls' test was used to compare percentages of surviving primordial or growing follicles among treatments and days of culture. Because of the higher coefficient of variation observed in both follicle and oocyte diameters, Duncan's test was applied to compare treatments tested, whilst Student's t-test was used to compare means between days of culture. Differences between groups were considered significant when p<0.05 and results were expressed as mean ± standard deviation (SD).
Results
Effect of FSH and culture duration on follicular viability
The percentage of normal preantral follicles in both cultured and non-cultured ovarian cortex is shown in figure 1. A total of 1560 preantral follicles were analysed to verify follicular morphology.
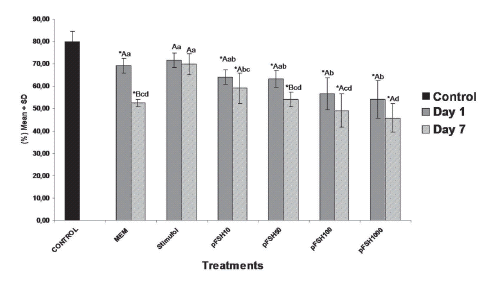
FIGURE1. Percentages (means ± SD) of histologically normal preantral follicles in non-cultured tissue (control) and in tissue cultured for one or seven days in medium with or without gonadotropin (either 50 ng/mL of Stimufol® or different concentrations of Folltropin®. *p<0,05, significantly different from non-cultured ovarian cortex tissue (control/D0). A,B Different letters on the same column denote significant differences between culture periods within the same dose (p<0.05). (a, b, c, d) Different letters on the same column denote significant differences between treatments in the same period (p<0.05).
After one or seven days of culture, a significant reduction (P < 0.05, Dunnett test) in the percentage of viable follicles was observed in the cultured control as well as in all Folltropin® treatments when compared to the non-cultured control. However, the percentage of viable follicles in Stimufol® treated samples (50 ng/mL), did not differ from the non-cultured controls (P>0.05, Dunnett test). Moreover, when comparisons were done among treatments, after seven days of culture, the highest percentage of viable follicles (P>0.05, SNK test) was observed with 50 ng/mL of Stimufol®. In addition to that, Folltropin® appeared to promote a dose dependent reduction in the number of viable follicles cultured samples exposed to 1000 ng/mL of Folltropin® showed a significantly lower percentage of viable follicles than those found in 10 ng/mL treated samples (P<0.05, SNK test). Also, after seven days of culture, there was a reduction (P < 0.05, SNK test) in the percentage of morphologically viable follicles in medium added with 50 ng/mL of Folltropin®, while all other Folltropin® treated groups did not differed significantly (P>0.05, SNK test) in the percentage of viable follicles from the cultured control.
Figure 2 shows the morphological integrity of oocytes and granulosa cells in caprine preantral follicles after seven days of culture with 50 ng/mL of Stimufol®.
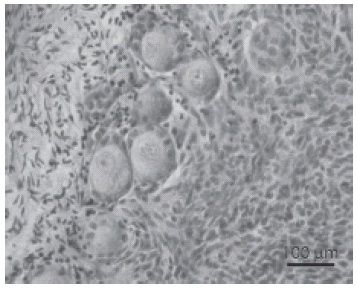
FIGURE 2. Histological section of culture tissue with Stimufol® 50 ng/ml (seven days of culture) after staining with periodic acid Schiff-hematoxylin, showing normal follicles. o: oocyte n: oocyte nucleus; gc: granulosa cells
Follicle activation during in vitro culture
The percentage of growing follicles in non-cultured and cultured control tissue was 16.7% and 23,1%, respectively, and the difference was not statistically significant (P>0.05, Dunnett test, Fig. 3). After one day of culture, there was a significant increase in the percentage of growing follicles (Fig. 3; P < 0.05, SNK test) in fragments treated with Folltropin® when compared to the cultured control. With the progression of the culture to seven days, an increase in the percentage of growing follicles (Fig. 3B; P<0.05, SNK test) was observed when the follicles were cultured in either medium alone or with 10 ng/mL of Folltropin®. In addition, when we compared the seven days treatments, tissues cultured with 100 ng/mL of Folltropin® showed a higher percentage of growing follicles (P<0.05, SNK test) as compared with medium alone or with 50 ng/mL of Stimufol®.

FIGURE 3. Percentages of growing follicles in noncultured tissues and in tissues cultured for one or seven days with or without gonadotropins addition (either 50 ng/mL of Stimufol® or different concentrations of Folltropin®). About 120 follicles were evaluated per treatment,. *p<0,05, significantly different from non-cultured ovarian cortex tissue (noncultured control and cultured tissue without gonadotropins). A,B Different letters on the same column denote significant differences between culture periods (1 vs 7 days) within the same dose (p<0.05; SNK test). a,b,c Different letters in the same column denote significant differences between treatments in the same period (p<0.05; SNK test).
Preantral follicle growth during in vitro culture
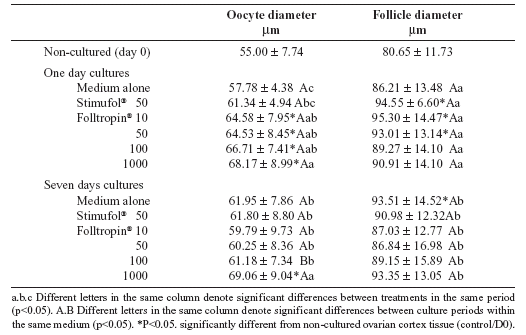
Follicular and oocyte diameters before and after the different culturing conditions are shown in Table 1. After seven days, a significant increase (P<0.05, Student's t test) in follicular diameter was observed only in follicles cultured with medium alone when compared to non-cultured tissue (control), however, there was not difference in follicular diameters among treatments. With the increase of the culture period from day one to day seven, there was a significant reduction (P < 0.05, Student's t test) in oocyte diameter after using of 100 ng/mL of Folltropin® (P < 0.05, Student's t test). At day seven, oocyte diameter was significantly higher after culture with 1000 ng/mL of Folltropin® when compared with all other treatments.
TABLE 1. Oocyte and follicle diameters (mean±SD) in non-cultured tissues and in tissues cultured for either one or seven days in control medium or after the addition of 50 ng/mL of Stimufol® and various concentrations of Folltropin® (ng/mL). Number of cases was 120 per group.
Ultrastructural analysis of caprine preantral follicles cultured in vitro
Based on histological results, transmission electron microscopy was performed in non-cultured follicles (control, Fig. 4A) and in follicles cultured for 7 days in MEM+ with 50 ng/mL of Stimufol® (Fig. 4B), which showed an ultrastructure similar to control. In both treatments, the follicle had intact basal and nuclear membranes, nucleus with descondensed chromatin and some vesicles. The organelles are uniformly distributed through the ooplasm, predominantly mitochondrias. Granulosa cells were ultrastructurally normal with an elongated nucleus and a high proportion nucleus-cytoplasm. It is important to note that we observed a slight detachment of the granulosa cells from the oocyte in follicles cultured in 50 ng/mL de Stimufol®.
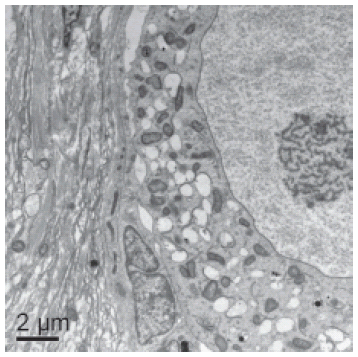
FIGURE 4. Ultrastructural images of (A) a non cultured preantral follicle (8000X) and (B) a follicle cultured for seven days in medium containing 50 ng/ml Stimufol (6000X). Note separation between granulosa cells and oocyte. n- nucleus, gc- granulosa cell, nc- nucleolus, m- mitochondria, v- vacuole, arrow- oocyte plasma membrane.
Discussion
One of the main factors that alter hormone bioactivity is the purity degree after its preparation (Closset and Hennen, 1989). In this study, two preparations of porcine pituitary FSH were evaluated on the viability and growth of caprine preantral follicles in vitro. Our results demonstrated that, after seven days, only the fragments of ovarian cortex cultured with Stimufol® had similar percentage of viable follicles when compared to control. On the other hand, all concentrations of Folltropin reduced the percentage of morphologically viable follicles after seven days. This result can be due to the fact that Stimufol® did not have significant amounts of LH activity contaminants. Although they are both porcine preparations, the proportion of contamination by LH (FSH:LH) is 20:1 in Stimufol® (Mazouz et al., 1996) and about 5,25:1 in Folltropin® (manufacturer's information). This difference in the FSH purity may due to differences in the hormonal purification technique between each laboratory. The same procedure for separation or purification of FSH can result in changes in the isoforms of this hormone (Bousfield et al., 2008). This suggests that the isoforms are influenced by the presence or absence of other proteins, such as LH. The current study showed that as higher the purity degree of FSH, as better the effectiveness of the preparation for the maintenance of caprine preantral follicles viability in an in vitro culture system. In another study, LH alone or in association with FSH resulted in degeneration of caprine preantral follicles cultured in vitro (Saraiva et al., 2008). Some authors have shown that Stimufol® (Tsai-Turton and Luderer, 2006; Matos et al., 2007) maintain viability and inhibit apoptosis of preantral follicles after in vitro culture in different species. Notwithstanding the beneficial effects of FSH on the in vitro follicular development, Nuttinck et al. (1996) have shown that pFSH (0.43 pg FSH/pg proteins) induces degeneration in small isolated bovine preantral follicles. These contradictions are likely due to the species, purity and hormone concentration differences, as well as in culture methodologies.
In the present study, we verify that addition of 10 ng/mL of Folltropin®, although did not provide high follicular viability rates, was capable to provide satisfactory activation rates in relation to Stimufol from day one to seven of culture. The inconstant ratio between FSH/LH in the commercial preparations of pFSH used, in part, can explain the results obtained in this study. It is known that FSH acts in early folliculogenesis (Joyce et al., 1999; Nilsson and Skinner, 2001) and is essential to an adequate development up to the preovulatory stage, however, the role of LH in this phase is not clearly defined (Salvetti et al., 2007). Studies have demonstrated that Folltropin® provides satisfactories results in the superovulation and embryo transfer protocols in different species (ovine: Cognie, 1999; caprine: Baldassarre and Karatzas, 2004; bovine: Bényei and Barros, 1999). On the other hand, Beckers (1987) showed that the reduction in LH content in the commercial preparations of gonadotrophin (FSH/LH: 12,5) is correlated with the reduction of the number of bovine embryo recovered by donor, although the embryo quality has improved. However, there is no studies about the effect of Folltropin® on the in vitro culture of preantral follicles.
Regarding follicular growth, we verified that after seven days, oocyte diameter was significantly higher after culture with 1000 ng/mL of Folltropin® when compared with the other treatments. Oktay et al. (1997) reported that the expression of FSH receptors developed progressively during the transition from primordial to primary and secondary follicles. The presence of FSH receptors in granulosa cells suggests that FSH can promote follicular development and growth. On the other hand, in our study, Folltropin® at a concentration of 100 ng/mL decreased follicular diameter from day one to seven of culture. We suggest that this occurs due to oocyte degeneration during the culture period, since in the preantral phase, the oocyte is more sensitive to degeneration than granulosa cells (Silva, et al., 2002).
Transmission electron microscopy was used as a qualitative and supplementary technique to evaluate follicular integrity after in vitro culture. In the present study, preantral follicles from non-cultured (control) fragments and from those cultured during seven days with Stimufol® were ultrastructurally normal, thus confirming the results obtained in the histological studies. It is important to emphasize that follicles cultured with Stimufol® for seven days had a slight separation between the oocyte and granulosa cells. This finding was also observed in swine preantral follicles cultured in vitro (Lucci, personal communication), which did not necessarely indicates follicular degeneration.
In conclusion, FSH preparations affect differently the performance of in vitro culture of caprine preantral follicles. Stimufol® was better to preserve follicular viability while Folltropin® was more efficient to promote follicular activation and oocyte growth.
Aknowledgments
Deborah de Melo Magalhães is a recipient of a grant from CNPq (Brazil). This work was supported by CNPq. The authors thank Tecnopec and Dr. J.F. Beckers for the kindly donation of Foltropin and Stimufol, respectively, tested in this study.
References
1. Baldassarre H, Karatzas CN (2004). Advanced assisted reproduction technologies (ART) in goats. Animal Reproduction Science 82-83: 255-266. [ Links ]
2. Beckers JF (1987). Isolation and use of a porcine FSH to improve the quality of superovulation in cattle. Theriogenology 27: 213. [ Links ]
3. Bényei B, Barros CCW (1999). Efeito da superovulação sobre o desempenho de bovinos doadores de embrião importado de clima temperado para clima tropical nos dois primeiros anos de adaptação. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 52: 366-371. [ Links ]
4. Bonnet-Garnier A, Lacaze S, Beckers JF, Berland HM, Pinton A, Yerle M, Ducos A (2008). Meiotic segregation analysis in cows carrying the t(1;29) Robertsonian translocation. Cytogenetic and Genome Research 120: 91-96. [ Links ]
5. Bousfield GR, Butnev VY, Bidart JM, Dalpathado D, Irungu J, Desaire H (2008). Chromatofocusing Fails To Separate hFSH Isoforms on the Basis of Glycan Structure. Biochemistry 47: 1708-1720. [ Links ]
6. Calder MD, Caveney AN, Smith LC, Watson AJ (2003). Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reproductive Biology and Endocrinology 14: 1-12. [ Links ]
7. Cecconi S, Barboni B, Coccia M, Mattioli M (1999). In vitro development of sheep preantral follicles. Biology of Reproduction 60: 594-601. [ Links ]
8. Closset J, Hennen G (1989). Biopotency of higly purified porcine FSH and human LH on gonadal function. Journal of Endocrinology 120: 89-96. [ Links ]
9. Cognie Y (1999). State of the art in sheep-goat embryo transfer. Theriogenology 51:105-116. [ Links ]
10. Eppig JJ (2001). Oocyte control of ovarian follicular development and function in mammals. Reproduction 122: 829-838. [ Links ]
11. Fiona TH, Ethier JF, Shimasaki S, Vanderhyden BC (2005). Follicle-Stimulating Hormone Regulates Oocyte Growth by Modulation of Expression of Oocyte and Granulosa Cell Factors. Endocrinology 146: 941-949. [ Links ]
12. Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R (2000). Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biology of Reproduction 62: 1322-1328. [ Links ]
13. Hulshof SCJ, Figueiredo JR, Beckers JF (1994). Isolation and characterization of preantral follicles from foetal bovine ovaries. Veterinary Quarterly 16: 78-80. [ Links ]
14. Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ (1999). Oocyte regulation of Kit ligand expression in mouse ovarian follicles. Development Biology. 214: 342-53. [ Links ]
15. Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN (2002). Effects of culturemedium, serumtype, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biology of Reproduction 67: 1197-1203. [ Links ]
16. Matos MHT, Lima-Verde IB, Luque MCA, Maia Jr JE, Silva JRV, Celestino JJH, Martins FS, Báo SN, Lucci CM, Figueiredo JR (2007). Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote 15: 173-182. [ Links ]
17. Mazouz A, Lotfi N, Elaich R, Lakhdissi H, Hachi A, Elaide L (1996). La technique de transfert d'embryons bovins chez leséleveurs moyen d'accroître le progrès génétique. Reproduction et production laitière: 271-277. [ Links ]
18. McGee E, Spears N, Minami S, Hsu SY, Chun SY, Billig H, Hsueh AJW (1997). Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3_-5_-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology 138: 2417-24. [ Links ]
19. Nilsson EE, Skinner MK (2001). Cellular interactions that control primordial follicle development and folliculogenesis. Journal of the Society for Gynecologic Investigation 8: 17-20. [ Links ]
20. Nuttinck F, Collete L, Massip A (1996). Histologic and autoradiographic study of the in vitro effects of FGF-2 and FSH on isolated bovine preantral follicles: preliminary investigations. Theriogenology 45: 1235-1245. [ Links ]
21. Oktay K, Briggs D, Gosden RG (1997). Ontogeny of follicle-stimulating hormone receptor gene expression in solated human ovarian follicles. Journal of Clinical Endocrinology & Metabolism 82: 3748-3751. [ Links ]
22. O'Shaughnessy PJ, Dudley K, Rajapaksha WR (1996). Expression of follicle stimulating hormone-receptor mRNA during gonadal development. Molecular and Cellular Endocrinology 125: 169-175. [ Links ]
23. Salvetti P, Theau-Clément M, Beckers JF, Hurtaud J, Guérin P, Neto V, Falières J, Joly T (2007). Effect of the luteinizing hormone on embryo production in superovulated rabbit does. Theriogenology 67: 1185-1193. [ Links ]
24. Saraiva MVA, Celestino JJH, Chaves RN, Martins FS, Bruno JB, Lima-Verde IB, Matos MHT, Silva GM, Porfirio EP, Báo SN, Campello CC, Silva JRV, Figueiredo JR (2008). Influence of different concentrations of LH and FSH on caprine primordial ovarian follicle development in vitro. Small Ruminant Research 78: 87-95. [ Links ]
23. Silva JRV, Ferreira MAL, Costa SHF, Santos RR, Carvalho FCA, Rodrigues APR, Lucci CM, Báo SN, Figueiredo JR (2002). Degeneration rate of preantral follicles in the ovaries of goats. Small Ruminant Research 43: 203-209. [ Links ]
24.Tecnopec (2008). http://www.tecnopec.com.br/index.php?aid=51. Accessed March 5, 2008. [ Links ]
25. Tsai-turton M, Luderer U (2006). Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 147: 1224-1236. [ Links ]
26. Ulloa-Aguirre A, Timossi C, Barrios-de-Tomasi J, Maldonado A, Nayudu P (2003). Impact of carbohydrate heterogeneity in function of FSH: studies derived from in vitro and in vivo models. Biology of Reproduction 69: 379-389. [ Links ]
27. Wright CS, Hovatta O, Margara R, Trew G, Winston RML, Franks S, Hardy K (1999). Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Human Reproduction 14: 1555-1562. [ Links ]
Received: July 10, 2008.
Revised version received: April 27, 2009.
Accepted: May 31, 2009.














