Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.33 n.2 Mendoza mayo/ago. 2009
ORIGINAL ARTICLES
Storage lipids and proteins of Euterpe edulis seeds
Vïctor Panza1,2, Dario Pighin3, Verónica Láinez2, Ricardo J. Pollero4, and Sara Maldonado1,2,*
1. Instituto de Recursos Biológicos CIRN, INTA. Las Cabañas y Los Reseros s/n. B1712WAA, Hurlingham. Buenos Aires, Argentina.
2. Departamento de Biodiversidad y Biología Experimental. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Pabellón 2, Ciudad Universitaria, C1428EHA, Ciudad Autónoma de Buenos Aires, Argentina.
3. Instituto de Tecnología de los Alimentos (INTA). Las Cabañas y los Reseros. CC 77 B1708WAB, Morón, Argentina.
4. Instituto de Investigaciones Bioquímicas de La Plata. CONICET-UNLP. Calles 60 y 120, La Plata, Argentina.
*Address correspondence to: Sara Maldonado. Depto. de Biodiversidad y Biología Experimental. Facultad de Ciencias Exactas y Naturales. UBA. Pabellón 2, Ciudad Universitaria. C1428EHA, Ciudad Autónoma de Buenos Aires, ARGENTINA. E-mail: saram@bg.fcen.uba.ar
ABSTRACT: Comparative studies on fatty acid and protein composition of the endosperm and embryo of palmito (Euterpe edulis Martius) were conducted using gas-liquid chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. On a dry weight basis, the embryo contained extremely lower amounts of lipids and proteins than did the endosperm, which was associated with the scarce lipid and protein bodies previously reported in axis and cotyledon. The fatty acid composition also exhibited differences between both tissues: (I) the fatty acid diversity was greater in embryo than in endosperm; (II) embryo and endosperm contained predominantly linoleic, palmitic, oleic and stearic acids even though the relative values were different for each tissue. As compared to other palm species, the higher fatty acid unsaturation in Euterpe edulis seed could be involved in the previously reported short longevity and recalcitrant behavior during storage. Proteins of both tissues were heterogeneous in molecular mass. Some proteins were tissue-specific, but other were common, among them a highly glycosylated protein which migrated at about 55 kDa. We hypothesize that the latter, also reported in all previously studied palm species, is one of the proteins characterizing the Arecaceae family.
Key words: Palm seeds; Arecaceae; Fatty acids; Recalcitrant behavior; Storage susceptibility.
Introduction
Seed studies carried out in Phoenix dactylifera L., Washingtonia filifera (Lindl.) Wendl. and Elaeis guineensis Jacq. show that the two areas of food reserves in a palm seed are the massive, hard endosperm and the small embryo (Alang et al., 1988; DeMason 1985; 1986; 1988; DeMason et al., 1983; 1989a, b; DeMason and Thomson, 1981; Meier, 1958; Meier and Reid, 1982). The stored reserves in palm endosperm are mannans, in the thickened cell walls, and lipid, protein, and mineral nutrients, in the cytoplasm. Lipids and proteins are in the form of lipid and protein bodies, and minerals are stored as phytin in the form of globoid crystal inside protein bodies. In the palm embryo, stored reserves also consist of lipids, proteins in the forms of lipid and protein bodies, the latter including phytin globoid crystals.
Differing from those three palm species, Panza et al. (2004) report that Euterpe edulis embryo cells have scarce storage reserves and exhibit an active state, with numerous mitochondria, rough endosperm reticulum cisternae, and Golgi apparatus, indicating a strategy of continuous development without the interposition, at maturity, of a dry state. In addition, that study indicates that whole embryo with 85% water content constitutes only 0.54% of the seed fresh weight, while that of the endosperm with 48.2% water content constitutes approximately 99% of the seed fresh weight.
Euterpe edulis seeds are short-lived and have recalcitrant storage behavior (Andrade, 2001; De León, 1961; Graziano, 1982; Martins et al., 2000; Reis et al., 1999), the latter feature differentiating this species from Phoenix dactylifera and Washingtonia filifera, both with orthodox seeds (Carpenter and Ream, 1976; Dickie et al., 1992; Krigman, 1974; Nixon, 1964; Sento, 1972) and also from Elaeis guineensis, in which seeds have an intermediate behavior (Ellis et al., 1991).
A difficulty in the interpretation of the experimental results on recalcitrant seeds is the differential water content between the embryo axis (and embryo) and the storage tissues (Berjak et al., 1989). In this respect Panza et al. (2007) demonstrate the need to study in separated form the embryo of the endosperma in E. edulis seeds.
To date, triacylglicerol seed composition has been studied only in two Arecaceae species, Elaeis guinensis (Salunkhe and Desai, 1986) and Cocos nucifera (Satyabalan, 1989). In both of them, the triacylglicerol composition is quite comparable, containing predominantly lauric (12:0), myristic (14:0), and palmitic (16:0) acids. Up to date, triacylglicerol composition of embryo and endosperm separately has not been reported for any palm species of Arecaceae.
Although palm endosperms are approximately 20% protein by weight, there is very little work on the characteristics of palm seed storage proteins. Previous studies, carried out by Chandra Sekhar and DeMason (1988a, b; 1989; 1990), DeMason and Chandra Sekhar (1990), and DeMason et al. (1985, 1989a) in Phoenix, Washingtonia, and Cocos, show that proteins are heterogeneous in molecular mass and charge ranging from 12 to 67 kDa and 3 to 10 in pI values. A number of common proteins exist in those genera which include both 7S and 11S globulins. In the three genera, the same authors have detected a highly glycosylated protein which migrates at about 55 kDa. They have also detected some individual differences between the three genera studied in relation to the relative quantities of the 67 kDa -7S globulin and 35 kDa -11S globulin. More recently García et al. (2005) determines in coconut that the basic polypeptide of the 11S globulin, which migrates at approximately 24 KDa, is glycosylated.
We have carried out a chemical study of the Euterpe edulis endosperm and embryo in which fatty acid and protein composition were analysed. This is the first chemical study in an embryo of the Arecaceae family. E. edulis is a tropical species occurring in a narrow range of rain forest in the Southern and Southeastern Brazil, Northeastern Argentina and Paraguay (Silva Matos and Watkinson, 1998). Its economical value is related to the production of "heart of palm", i.e. the growing apical bud surrounded by young leaves (Nodari and Guerra, 1986). Most of the time the plants are harvested before they reach maturity and produce seeds, the only propagation method of this species. The economical management has been conducted in an essentially predatory way and that represents a threat to the survival of this species. This study constitutes part of a monographic treatment on conservation of Euterpe edulis.
Material and methods
Plant material
Mature fruits of Euterpe edulis Martius (Fig. 1A) were harvested from trees growing in Parque Nacional Iguazú, Provincia de Misiones Argentina, during the month of August for three consecutive years (2001 to 2003), and shipped by expedited post to the Bank of Germplasm of INTA, Castelar, Buenos Aires, Argentina, where studies were conducted.
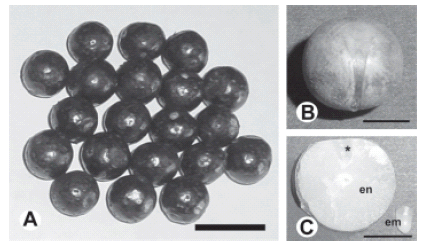
FIGURE 1. Euterpe edulis fruits and seeds: A, mature fruits; scale bar = 2 cm. B, seed cleaned of pericarp, as seen from the raphe: scale bar = 0.2 cm. C, longitudinal medial section trough a seed showing endosperm (en) and detached embryo (em). Asterisk indicates embryo position within the endosperm; scale bar = 0.2 cm.
Lipids and proteins were determined for embryo and endosperm tissues separately. For this purpose, both embryos and endosperms were removed from the seeds (Fig. 1C) and lyophilized.
Lipid extraction and fatty acid analysis
For fatty acid analysis, lyophilized and ground endosperm and embryo tissues were transferred into 1.5 ml eppendorf tubes and total lipids were extracted with chloroform - methanol mix using the procedure described by Folch et al. (1957). Total lipid extracts were dried and weighted, suspended in 2 ml of a fresh solution of 10% KOH in ethanol and saponified during 60 min at 80ºC using stopped glass tubes. Two ml hexane was added and fatty acids were extracted by shaking. The upper organic phase (non-saponifiable) was discarded. The aqueous layer was acidified with 1.5 ml of concentrated HCl and fatty acids were extracted twice with 1.5 ml hexane. Extracts containing free fatty acids were dried under a nitrogen stream, dissolved in 1.5 ml BF3 (10% in methanol) and 1.5 ml benzene and esterified by heating and shaking at 100ºC for 1 h. Fatty acid methyl esters were extracted twice with hexane and washed with distilled water. After washing, the organic phase was evaporated under a nitrogen stream, re-dissolved in hexane, and analyzed by gas-liquid chromatography. One μl of the fatty acid methyl esters solution was injected into an Omegawax X250 (Supelco Inc., Bellefonte, Pennsylvania) capillary column (30 m x 0.25 mm, 0.25 μm film) in a Hewlett Packard HP-6890 chromatograph equipped with a flame ionization detector. The column temperature was programmed for a linear increase of 3ºC/min from 175 to 230ºC. The chromatographic peaks of fatty acid methyl esters were identified by comparison of their retention times with standards, under similar conditions.
Protein extraction and protein analysis
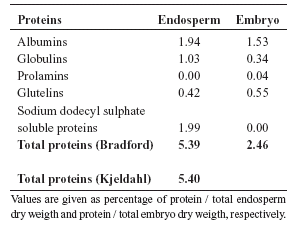
Total protein content was determined in triplicate for each year's collection (three years 2001-2003) by the Kjeldahl's method, using the value of 5.7 as conversion factor. After lipid extraction, the dried pellet was used to obtain different fractions of the major storage proteins for quantification using bovine serum albumin as standard (Bradford, 1976), as follows:
(I) To obtain water-soluble albumins, pellets were re-suspended in 50 mM Tris-HCl, pH 8.3, 1 μM benzamidine, 1 μM phenyl-methylsulfonyl fluoride in the ratio of 10 μl/mg of sample. Samples were stirred for 30 minutes, centrifuged for 10 minutes at 10,000 g and the supernatant collected as soluble fractions.
(II) To obtain salt-soluble globulins, pellets after low-salt extraction were re-suspended in 50 mM Tris-HCl, 1.0 M NaCl, 1 μM benzamidine, 1 μM phenylmethylsulfonyl fluoride. After stirring for 60 minutes, the samples were centrifuged for 10 minutes at 10.000 g and the supernatant collected as a high salt fraction.
(III) To obtain prolamins, pellets were re-suspended in ethanol 70% at 65ºC, 1 μM benzamidine, 1 μM phenyl-methylsulfonyl fluoride. After stirring for 30 minutes, the samples were centrifuged for 10 minutes at 10.000 g and the supernatant collected as prolamins fraction.
(IV) To obtain glutelins, pellets were re-suspended in NaOH 0.1 M, 1 μM benzamidine, 1 μM phenylmethylsulfonyl fluoride. After stirring for 30 minutes, the samples were centrifuged for 10 minutes at 10,000 g and the supernatant collected as glutelins fraction.
(V) The remaining proteins were obtained as follows: pellets after NaOH extraction were re-suspended in 50 mM Tris-HCl, 2% sodium dodecyl sulfate. The samples were stirred for 60 minutes, and then centrifuged for 10 minutes at 10,000 g. The supernatant was collected as remainder fractions. This procedure was repeated twice to ensure that all proteins were removed from the ground material.
Polyacrylamide gel electrophoresis under denaturation conditions was conducted according to Laemmli (1970) with some modifications. Electrophoresis was run in a linear gradient of acrylamide concentration (8-15%) in a Mini Protean III system (Bio-Rad), at constant voltage (130 V) for 90 min. Glycerol (5% v/v) was added to both stacking and resolving gel solutions. For analytical purposes, 40 μg proteins were loaded onto each well. Low Range Molecular Weight calibration stained kit (Bio-Rad) was used to estimate the molecular weight (MW) of the different proteins. Gels were stained with 0.1% (w/v) Coomasie Brilliant Blue R-250 solution, and then analyzed with a Bio-Rad GS-800 Imaging Calibrated Densitometer. Images were captured and processed by Quantity One 1-D Analysis software. For glycoprotein detection, gels were stained with the periodate-Schiff reaction (Segrest and Jackson, 1972).
Results and Discussion
This is the first study on triacylglicerol and proteins composition in an embryo of the Arecaceae family. Previous studies were made on whole seeds. On this respect, Grout et al. (1983) and Berjak et al. (1989) warn on the difficulty of comparing groups of data in which there are marked differences on size and water content between both tissues. The difficulty is exacerbated in Euterpe edulis where the whole embryo is minute (Fig.1C) and constitutes only 0.54% of the seed's fresh weight (Panza et al., 2004).
Fatty acid analysis
In E. edulis endosperm, total lipids represented around 0.45% of the total endosperm weight (dry weight basis). In the embryo, lipids were only present in extremely low quantity. Unsaturated fatty acids were predominant, the sum of which was around 65% of total fatty acids of endosperm and 60% of embryo. Endosperm and embryo contained predominantly linoleic (18:2ω6), palmitic (C16:0), and oleic (C18:1ω9) acids even though for each tissue the relative values were different (Fig. 2; Table 1). In embryo, a-linolenic (18:3 ω3) and palmitoleic (16:1 ω7) acid and stearic (C18:0) acid concentrations were almost similar. In endosperm, the a-linolenic and palmitoleic were minor fatty acids. The oleic isomer 18:1 ω7 (vacenic), biosynthetically derived from the palmitoleic acid, was also quantitatively important in embryo, but it is a minor fatty acid in endosperm. Traces of other fatty acids of 16, 20, 22, and 24 carbons were also detected in both tissues (Fig. 2; Table 1).

FIGURE 2. Fatty acid composition of Euterpe edulis endosperm and embryo as analyzed by gasliquid chromatography. The chromatographic peaks of fatty acid methyl esters were identified by comparison of their retention times with those of standards chromatographed under the same conditions. A, endosperm; B, embryo. Abbreviations: l, linoleic acid; ln, linolenic acid; m, myristic acid; o, oleic acid; p, palmitic acid; po, palmitoleic acid; s, stearic acid; v, vacenic acid.
TABLE 1. Percentages of the Euterpe edulis fatty acids.

Gurr (1980) reports that, even though fatty acids tend to be characteristic of particular plant families, they could vary widely among species. To date, the seed fatty acid composition in palms is known for two species, Elaeis guinensis and Cocos nucifera (Salunkhe and Desai, 1986; Satyabalan, 1989). In those two species, lauric, myristic, and palmitic (saturated) acids are predominant. Differing from those species both tissues in E. edulis predominantly contained (as mentioned above) linoleic, palmitic, and oleic acids. According to Miquel and Browse (1995) 18-carbon unsaturated and polyunsaturated fatty acids are generally predominant in Angiosperms. In endosperm and embryo of Euterpe edulis they represented around 55 and 48%, respectively. On this respect, Staehelin and Newcomb (2000) identify linoleic and linolenic acids (in addition to palmitic acid) as the main fatty acids in plants frequently associated with membrane construction.
Additionally, Euterpe edulis seeds are short-lived (De León, 1961; Graziano, 1982) and have recalcitrant storage behavior (Andrade, 2001; Martins et al., 2000; Reis et al., 1999), differentiating this species from Phoenix dactylifera and Washingtonia filifera, both with orthodox seeds (Carpenter and Ream, 1976; Dickie et al., 1992; Krigman, 1974; Nixon, 1964; Sento, 1972) and also from Elaeis guineensis, in which seeds have an intermediate behavior (Ellis et al., 1991). At present we cannot establish in the E. edulis embryo the biochemical events responsible for its short longevity and recalcitrant behavior, but it is known that polyunsaturated fatty acids, the most oxygen sensitive molecules encountered in nature, are present in membranes (Spiteller, 2003). The high fatty acid unsaturation evokes changes in membrane structures, commonly enhancing their fluidities. In addition, unsaturation also increases fatty acid susceptibility to degradation as a consequence of the double bond peroxidation. The short longevity found in E. edulis seed (De León, 1961; Graziano, 1982), which is only capable of initiating germination within a short time following shedding, could be reflecting the relative high content of unsaturated acids found in E. edulis seed (which represented around 65% and 60% of total endosperm and embryo fatty acid, respectively). These facts or some other property conferred by the higher fatty acid unsaturation in Euterpe edulis seeds, as compared to other palm species, would also contribute to explain its behavior during storage.
The fatty acid spectrum was wider in embryo than in endosperm, i.e. several 15- and 16-carbon fatty acids were detected only in embryos, but were absent in endosperm. Such diversity could be associated with the more active biosynthetic mechanisms to elongate and to desaturate fatty acids which are needed during germination.
Protein analysis
Up to now, very little is known about seed proteins from palm seeds. In fact, Chandra Sekhar and DeMason (1988a, b; 1989; 1990), DeMason and Chandra Sekhar (1990), and DeMason et al. (1985; 1989a) electrophoretically characterize seed proteins in Phoenix, Washingtonia, and Cocos, showing that they are heterogeneous in molecular mass and charge ranging from 12 to 67 kDa. A number of common proteins exist in all those genera which include both 7S and 11S globulins. The same authors detect a highly glycosylated protein which migrates at about 55 kDa in the three above mentioned genera. They also detect some differences between the three genera studied in relation to the relative quantities of the 67 kDa - 7S globulin and 35 kDa -11S globulin.
In Euterpe edulis total protein content, quantified by the Bradford's method, represented 5.39% and 2.46% of the endosperm and embryo dry weight, respectively (Table 2). Total protein endosperm content quantified by the Kjeldahl's method represented 5.4% of the endosperm dry weight. Total soluble proteins were also quantified for different fractions of both endosperm and embryo, showing that the two major fractions were albumins, globulins and sodium dodecyl sulphate-soluble proteins for endosperm, and albumins and glutelins for embryo (Table 2). The difference in protein quantity correlates well with very small quantities of protein bodies present in the embryo tissues (Panza et al., 2004), consequently, we infer that the source of proteins in embryos may also be various organelles and the organelle-free cytoplasm.
TABLE 2. Protein fractions and total proteins of endosperm and embryo of E. edulis.
The electrophoretic study of total soluble proteins in Euterpe edulis showed a different profile for both endosperm and embryo tissues (Fig. 3, lanes b and c). In Phoenix, Washingtonia and Cocos, the electrophoretic techniques show that the major seed proteins are not tissue-specific; this correlates with the histochemical similarity in the endosperm and embryo of these species with proper quiescent tissues. In the three genera, cells store abundant lipids and proteins in the form of lipid and protein bodies, the percentage of normal cell constituents (cytoplasm, nucleus and other organelles) is very small, and, at the transmission electron microscopy level, no ribosomes or endoplasmic reticulum or endomembranes are discernable in mature tissues (DeMason, 1986; 1988; DeMason and Thomson, 1981; DeMason et al., 1983).
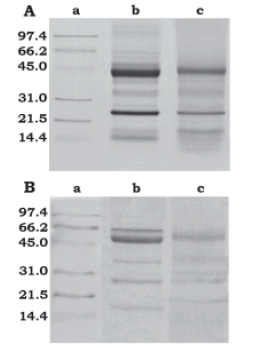
FIGURE 3. Sodium dodecyl sulphate polyacrylamide gel electrophoresis analysis of Euterpe edulis endosperm and embryo. For both gels: lane a = molecular weight standards; lane b= endosperm soluble proteins; lane c = embryo soluble proteins. A, gel was stained with 0.1% (w/v) Coomassie Blue-R-250; B, gel was stained with periodate-Schiff reaction (five major glycosylated endosperm bands, of approximately 16, 25, 35, 50 and 55 kDa and two major glycosylated embryo bands, of approximately 25 and 50 KDa were observed).
In E. edulis, endosperm proteins resolved into 13 main bands ranging between 14 and 141 kDa (Fig. 3A, lane b) and embryo proteins resolved into 7 main bands ranging from 17 to 45 kDa (Fig. 3A, lane c). Endosperm and embryo had in common bands of approximately 17, 22, 25, 28, 35, and 40 kDa. Bands between 50 and 141 kDa, were detected clearly in the endosperm but not in the embryo. Endosperm profile included both 67 kDa -7S globulin and 35 kDa - 11S globulin that are present in Phoenix, Washingtonia and Cocos (Chandra Sekhar and DeMason, 1988a, b; 1989a; DeMason and Chandra Sekhar, 1990).
Using the periodate-Schiff reaction (Fig. 3B), five major glycosylated bands were detected in endosperm of approximately 16, 25, 35, 50 and 55 kDa, and two bands in embryo, of approximately 25 and 50 KDa. The 25 KDa bands, which are present in both tissues, would correspond to cocosin, i.e. the basic component of the 11S globulin detected in coconut by García et al. (2005).
The 55 kDa glycosylated protein found in E. edulis endosperm, is present, according to DeMason (personal communication) not only in the endosperm of the three previously studied species (Phoenix, Washingtonia and Cocos) but also in all endosperm of other species she has analyzed briefly in her lab, i.e. species of the genera Calamus, Chamaedora, Caryota, Erythea and Syagrus. Establishing if the 55 kDa protein constitutes a taxonomic marker for the Arecaceae awaits further investigation in other palm species.
Acknowledgments
This work was supported by a grant from Agencia de Promoción Científica y Tecnológica (ANPCyT) (Argentina), PICT 1201/OC-AR 08-04536. We thank Justo Herrera and Karina Schiaffino, Centro de Investigaciones Ecológicas Subtropicales (CIES), Parque Nacional Iguazú, Argentina for help in obtaining specimens and field assistance. We thank Adriana Pazos Instituto de Tecnología de los Alimentos, INTA, Argentina, and Pilar Buera and Carolina Schebor from Laboratorio de Propiedades Fisico-Químicas de Biomateriales (Facultad de Ciencias Exactas y Naturales-Universidad de Buenos Aires), for lab work assistance.
References
1. Alang CA, Moir GFJ, Jones LH (1988). Composition, degradation and utilization of endosperm during germination in the oil palm (Elaeis guineensis Jacq.). Annals of Botany 61: 261. [ Links ]
2. Andrade ACS (2001). The effect of moisture content and temperature on the longevity of heart of palm seeds (Euterpe edulis). Seed Science Technology 29: 171-182. [ Links ]
3. Berjak P, Farrant JM, Pammenter NW (1989). The basis of recalcitrant seed behaviour. In: Recent advances in the development and germination of seeds (RB Taylorson, ed.), p. 89-108. Plenum Press, New York. [ Links ]
4. Bradford MM (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254. [ Links ]
5. Carpenter JB, Ream CL (1976). Date palm breeding, a review. Date Growers Institute Report 53: 25-33. [ Links ]
6. Chandra Sekhar KN, DeMason DA (1988a). Quantitative ultrastructure and protein composition of date palm (Phoenix dactylifera) seeds: a comparative study of endosperm versus embryo. American Journal of Botany 75: 323-329. [ Links ]
7. Chandra Sekhar KN, DeMason DA (1988b). A comparative study of endosperm and embryo proteins of the palm, Washingtonia filifera. American Journal of Botany 75: 338-342. [ Links ]
8. Chandra Sekhar KN, DeMason DA (1989). Differential activity of acid phosphatase from the endosperm and haustorium of date palm (Phoenix dactylifera L.) seeds. Canadian Journal of Botany 67:1096-1102. [ Links ]
9. Chandra Sekhar KN, DeMason DA (1990). Identification and immunocytochemical localization of á-galactosidase in resting and germinated date palm (Phoenix dactylifera L.) seed. Planta. 181: 53-61. [ Links ]
10. De Leon NJ (1961). Viability of palm seeds. American Horticultural Magazine 40: 131-132. [ Links ]
11. DeMason DA, Thomson WW (1981). Structure and ultrastructure of the cotyledon of date palm (Phoenix dactylifera L.). Botanical Gazette 142: 320-328. [ Links ]
12. DeMason DA, Sexton R, Reid JSG (1983). Structure, composition and physiological state of the endosperm of Phoenix dactylifera L. Annals of Botany 52: 71-80. [ Links ]
13. DeMason DA (1985). Histochemical and ultrastructural changes in the haustorium of date (Phoenix dactylifera L.). Protoplasma 126: 168-177. [ Links ]
14. DeMason DA, Sexton R, Gorman M, Reid JSG (1985). Structure and biochemistry of endosperm breakdown in date palm (Phoenix dactylifera L.) seeds. Protoplasma 126: 159-167. [ Links ]
15. DeMason DA (1986). Endosperm structure and storage reserve histochemistry in the palm, Washingtonia filifera. American Journal of Botany 73: 332-1340. [ Links ]
16. DeMason DA (1988). Embryo structure and storage reserve histochemistry in the palm, Washingtonia filifera. American Journal of Botany 75: 330-337. [ Links ]
17. DeMason DA, Madore MA, Harris M (1989a). Endosperm development in the date palm (Phoenix dactylifera) (Arecaceae). American Journal of Botany 76: 1255-1265. [ Links ]
18. DeMason DA, Stillman JI, Ellmore GS (1989b). Acid phosphatase localization in seedling tissues of the palms Phoenix dactylifera and Washingtonia filifera, and its relevance to control of germination. Canadian Journal of Botany 67: 1103-1110. [ Links ]
19. DeMason D, Chandra Sekhar KN (1990). Electrophoretic characterization and immunological localization of coconut (Cocos mucifera L.) endosperm storage proteins. Botanical Gazette 151: 302-313. [ Links ]
20. Dickie JB, Balik MJ, Linnington IM (1992). Experimental investigations into feasibility of ex-situ preservation of palm seeds; an alternative strategy for biological conservation of this economically important plant family. Biodiversity and Conservation 1: 112-119. [ Links ]
21. Ellis RH, Hong TD, Roberts, EH, Soetisna U (1991). Seed storage behaviour in Elaeis guineensis. Seed Science Research 1: 99-104. [ Links ]
22. Folch J, Lees M, Sloane-Stanley GH (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226: 497-509. [ Links ]
23. García RN, Arocena RV, Antonio C. Laurena AC, Tecson-Mendoza EM (2005). 11S and 7S Globulins of Coconut (Cocos nucifera L.): Purification and Characterization. Journal of Agricultural and Food Chemistry 53: 1734-1739. [ Links ]
24. Graziano TT (1982) Viability of Palm Seeds 1. Euterpe edulis and Ptychosperma macarthurii. Científica (Jaboticabal) 10: 273-276. [ Links ]
25. Grout BWW, Shelton K, Pritchard HW (1983). Orthodox behaviour of oil palm seed and cryopreservation of the excised embryo for genetic conservation. Annals of Botany 52: 381-384. [ Links ]
26. Gurr MI (1980). The Biosynthesis of Triacylglicerols. In: The Biochemistry of Plants (PK Stumpf, ed.), p. 205-246. Academic Press, New York. [ Links ]
27. Krigman SL (1974). Washingtonia filifera (Linden) Wendl. Seeds of Woody Plants in the United States. Agriculture Handbook 450: 855-856. [ Links ]
28. Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [ Links ]
29. Martins CC, Nakagawa J, Alves Bovi ML (2000). Desiccation tolerance of four seed lots from Euterpe edulis Mart. Seed Science and Technology 28: 101-113. [ Links ]
30. Meier H (1958). On the structure of cell walls and cell wall mannans from ivory nuts and from dates. Biochimica et Biophysica Acta 28: 229-240. [ Links ]
31. Meier H, Reid JSG (1982). Reserve polysaccharides other than starch in higher plants. In: Encyclopaedia of Plant Physiology, New Series, Vol. 13A. Plant Carbohydrates I. (FA Loewus, W Tanner, eds.), p. 418-471. Springer-Verlag, Berlin. [ Links ]
32. Miquel MJ, Browse J (1995). Lipid byosinthesis in developing seeds. In: Seed development and germination (J Kigel, G Galili, eds.), p. 169-193. Dekker, New York. [ Links ]
33. Nixon RW (1964). Viability of date seed in relation to age. Date Growers Institute Report 41: 3-4. [ Links ]
34. Nodari RO, Guerra MP (1986). Palmito in Southern Brazil: Status and perspectivas. Newsletter: Useful palms of Tropical America 2: 9-10. [ Links ]
35. Panza V, Láinez V, Maldonado S (2004). Seed Structure and Histochemistry in the Palm Euterpe edulis. Botanical Journal of the Linnean Society 145: 445-453. [ Links ]
36. Panza V, Láinez V, Maroder HL, Prego I, Maldonado S (2007). Effects of desiccation on "palmito" (Euterpe edulis Mar.) seeds. Biocell 31: 383-390. [ Links ]
37. Reis A, Silveira Paulilo MT, Nakazono EM, Venturi S (1999). Effect of different level of desiccation in the seed germination of Euterpe edulis Martius - Arecaceae. INSULA 28: 31-42. [ Links ]
38. Salunkhe DK, Desai BB (1986). Postharvest Biotechnology of oil seeds. CRC Press, Boca Raton. [ Links ]
39. Satyabalan K (1989). Coconut. Oil Crops of the World. McGraw-Hill, New York. [ Links ]
40. Segrest JP, Jackson RL (1972). Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis in sodium dodecyl sulphate. Method Enzymol 28: 54-63. [ Links ]
41. Sento T (1972). Studies on the seed germination of palm. V. On Chrysalidocarpus lutescens, Mascarena verschaffeltii and Phoenix dactylifera. Journal of the Japanese Society for Horticultural Science 41: 76-82. [ Links ]
42. Silva Matos DM, Watkinson AR (1998). The fecundity, seed, and seedling ecology of the edible palm Euterpe edulis in southeastern Brazil. Biotropica 30: 595-603. [ Links ]
43. Spiteller G (2003). The relationship between changes in the cell wall, lipid peroxidation, proliferation, senescence and cell death. Physiologia Plantarum 119: 5-18. [ Links ]
44. Staehelin LA, Newcomb EH (2000). Membrane structure and membranous organelles. In: Biochemistry and molecular biology of plants (B Buchanan, W Gruissem, R Jones, eds.), p 2-50. American Society of Plant Physiology, Rockville. [ Links ]
Received: September 24, 2008.
Accepted: April 19, 2009.














