Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell v.33 n.2 Mendoza maio/ago. 2009
ORIGINAL ARTICLES
Simvastatin acts as an inhibitor of interferon gamma-induced cycloxygenase-2 expression in human THP-1 cells, but not in murine RAW264.7 cells
Chang Seok Lee1, Yong Jae Shin1, Cheolhee Won1, Yun-Song Lee2, Chung-Gyu Park3, Sang-Kyu Ye1* and Myung-Hee Chung1
1. Department of Pharmacology, Seoul National University College of Medicine, Seoul 110-799, Korea.
2. Department of Pharmacology, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea.
3. Department of Microbiology, Seoul National University College of Medicine, Seoul 110-799, Korea.
*Address correspondence to: Sang-Kyu Ye. Department of Pharmacology, Seoul National University College of Medicine, Seoul 110-799, KOREA. E-mail: sangkyu@snu.ac.kr
ABSTRACT: Cyclooxygenase-2 (COX-2) is a key inflammatory response molecule, and associated with many immune functions of monocytes/macrophages. Particularly, interferon gamma (IFNg)-induced COX-2 expression appears in inflammatory conditions such as viral infection and autoimmune diseases. Recently, statins have been reported to show variable effects on COX-2 expression, and on their cell and species type dependences. Based on the above description, we compared the effect of simvastatin on IFNg-induced COX-2 expression in human monocytes versus murine macrophages. In a result, we found that simvastatin suppresses IFNg-induced COX-2 expression in human THP-1 monocytes, but rather, potentiates IFNg-induced COX-2 expression in murine RAW264.7 macrophages. However, signal transducer and activator of transcription 1/3 (STAT1/3), known as a transcription factor on COX-2 expression, is inactivated by simvastatin in both cells. Our findings showed that simvastatin is likely to suppress IFNg-induced COX-2 expression by inhibiting STAT1/3 activation in human THP-1 cells, but not in murine RAW264.7 cells. Thus, we concluded that IFNg-induced COX-2 expression is differently regulated by simvastatin depending on species specific mechanism.
Key words: Monocyte; Macrophage; INFg; STAT1/3; SOCS1/3
Introduction
Cyclooxygenase-2 (COX-2) is the rate limiting enzyme in the conversion of arachidonic acid into prostanoids. In monocytes and macrophages, prostaglandins E2 and thromboxane A2, the major products of COX-2, modulate inflammatory signs. Therefore, COX-2 is highly expressed in inflamed tissues and is considered as a key molecule in inflammatory environments. For COX-2 expression, the NF-κB and signal transducer and activator of transcription (STAT) binding elements of COX-2 gene promoter are known to participate in transcription (Inoue and Tanabe, 1998; Xuan et al., 2003).
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are used to treat hypercholesterolemia, and are known to have beneficial effects on vascular atherosclerosis, stroke, heart transplantation, and Alzheimer disease patients (Liao, 2004; Tunon et al., 2007; Kobashigawa, 2004; Miller and Chacko, 2004). Recent studies have shown that statins, like simvastatin, have immuno-modulatory and anti-inflammatory effects (Chello et al., 2007; Chen et al., 2007; Greenwood and Mason, 2007). Several groups have suggested that statins have anti-inflammatory effects, for example, they inhibit the productions of proinflammatory cytokines and the expressions of major inflammatory molecules (Grip et al., 2000; Ortego et al., 1999). Furthermore, it has been reported that the lovastatin and fluvastatin inhibit inducible nitric oxide synthase (iNOS) expression by blocking NF-κB and STAT signaling in murine macrophages (Huang et al., 2003). It has also been reported that simvastatin inhibits IFNg-induced major histocompatibility complex class II (MHC-II) molecules that are expressed on the surfaces of various cells (Ghittoni et al., 2006), and another report found that simvastatin suppresses IFNg-induced CD40 expression in murine macrophages (Lee et al., 2007). These anti-inflammatory effects of statins usually depend on the reduction of mevalonate (the direct product of HMG-CoA reductase) and mevalonatederived isoprenoids, such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate.
Recently it has been reported that simvastatin blocks COX-2 expression in human monocyte U937 cells in a Rac and NF-κB dependent manner (Habib et al., 2007). However, mevastatin and lovastatin were found to increase COX-2 expression by inhibiting Rho A in human aortic smooth muscle cells (Degraeve et al., 2001; Monick MM et al., 2003), and furthermore, fluvastatin, atorvastatin and lovastatin were also found to induce COX-2 expression via a MAPK cascade dependent pathway in murine macrophages (Chen et al., 2004). Thus, statins appear to have capricious effects on COX-2 expression.
In the present study, to elucidate the effect of simvastatin on IFNg-induced COX-2 expression in human monocytes and murine macrophages, we performed experiments on THP-1 cells (human monocytes) and RAW264.7 cells (murine macrophages). It was found that in IFNg-stimulated human THP-1 cells, simvastatin inhibits COX-2 expression inducing suppressor of cytokine signaling 1/3 (SOCS1/3) which prevents STAT1/3 activation. However, paradoxically, simvastatin potentiated COX-2 expression in IFNg-stimulated murine RAW264.7 cells, despite inducing SOCS1/3 and inhibiting STAT1/3 activation. These findings suggest that the effects of simvastatin on IFNg-induced COX-2 expression may vary depending on cell and/or species type.
Materials and Methods
Reagents and materials
Simvastatin, which was purchased in the inactive lactone, was hydrolyzed to its active hydroxyacid as described below. This form is referred to throughout as simvastatin. Simvastatin was prepared as a 20 mM stock solution, by dissolving 8.37 mg of the inactive lactone in 100 μl of ethanol and 150 μl of 0.1 N NaOH and incubating at 50ºC for 2 h. The pH was then adjusted to 7.0, and the total volume was made up to 1 ml. This simvastatin stock solution was diluted with sterile phosphate buffer-saline immediately before use.
In all experiments, we used simvastatin at 2 μM. Recombinant human IFNg was purchased from Biosource International (CA, USA) and used at 50 ng/ml. Antibodies against COX-2 and inducible nitric oxide synthase (iNOS) were purchased from ABcam (Cambridge, UK), and antibodies against actin, Tyr-701-phosphorylated STAT1, and Tyr-705-phosphorylated STAT3 were purchased from Santa Cruz Biotechnology (CA, USA).
Cell lines and primary macrophage culture
THP-1 cells (a human monocytic leukemia cell line) were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum at 37ºC and 5% CO2, whereas RAW 264.7 cells (a murine macrophage cell line) and BV2 cells (a murine microglia cell line) were maintained in DMEM supplemented with 10% fetal bovine serum at 37ºC and 5% CO2. For experiments using primary murine macrophages, primary peritoneal cells were obtained from C57BL6 male mice. Briefly, mice were injected with 1 ml of 3% thioglycolate media intraperitoneally (i.p.), and 4 days later thioglycolate elicited peritoneal cells were harvested by lavage with 10 ml of phosphate buffer-saline. Cells were plated on 60 mm dishes in RPMI1640 medium containing 10% fetal bovine serum at 37ºC and 5% CO2 and incubated for 4 h. Dishes were then washed with phosphate buffer-saline to remove nonadherent cells and the adherent cells so obtained were used as peritoneal macrophages.
Cell cytotoxicity assays
They were performed using the Cell Counting Kit-8, as described by the manufacturer (DOJINDO, Tokyo). Briefly, THP-1 and RAW264.7 cells (0.5x104 cells) were seeded in 96-well culture plates and incubated for 24 h at 37ºC and 5% CO2. Then, cells were incubated with simvastatin indicated dosage or time. Finally, CCK-8 solution was added to each well of plate for 4 h, then, we measured the absorbance at 450 nm using a microplate reader.
Western blot analysis
Cells were washed twice with cold PHOSPHATE BUFFER-SALINE, and then lysed in ice-cold modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl) containing protease inhibitors (2 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 0.5 mM Na3VO4, 2 mM EDTA and 1 mM NaF). Lysates were centrifuged at 13,000 rpm for 20 min at 4ºC, and supernatants were collected. Proteins in lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies, washed, treated with peroxidase-conjugated secondary antibodies, rewashed, and then visualized using an Amersham ECL system (NJ, USA). COX-2 bands were quantified and normalized versus actin bands using ImageJ version 1.35d (National Institute of Health Imaging software).
Reverse transcription-polymerase chain reaction (RTPCR)
Total RNA was extracted from cell lysates using Easy-BLUE (iNtRON, Seoul), and cDNA was prepared using a Maxime RT-premix (iNtRON, Seoul), according to the manufacturer's instructions. PCR was performed over 30 cycles of 94ºC for 60 s, 60ºC for 60 s, and 72ºC for 120 s. Oligonucleotide primers were purchased from Bioneer (Seoul). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control "housekeeping gene". PCR products were separated by electrophoresis in 1% agarose gels and detected under UV light. PCR primer sequences are detailed in Table 1.
TABLE 1. Sequences of RT-PCR oligonucleotide primers.

Results
Simvastatin suppresses the expression of COX-2 and costimulatory molecules in IFNg-stimulated THP-1 cells
Prior to examining the effect of simvastatin on IFNg-induced COX-2 expression, we investigated the cytotoxic effect of simvastatin on THP-1 and RAW264.7 cells. However, simvastatin was found to have no cytotoxicity effect after 24 h at concentrations of 0.1-2 μM. To examine its effect versus time, simvastatin was treated at 2 μM for indicated time, but no effect was observed at 48 h both THP-1 (Fig. 1A) and RAW264.7 cells (data not shown). Then, to investigate the effect of simvastatin on IFNg-induced COX-2 expression, THP-1 cells were pre-treated for 6 h with simvastatin and treated with 50 ng/ml IFNg for 18 h. Cell lysates were subjected to Western blot analysis to determine COX-2 protein levels. The results obtained showed that simvastatin suppressed COX-2 expression in these IFNg-stimulated cells (Fig. 1B). Next, we tested immunomodulatory effect of simvastatin in IFNg-stimulated THP-1 cells because statins are known to have inhibitory effect on the expression of cell surface molecules such as ICAM-I, CD40 and CD86 (Chung et al., 2002; Ghittoni et al., 2006; Lee et al., 2007; Kuipers et al., 2005; Sadeghi et al., 2001). THP-1 cells were pre-treated for 6 h with simvastatin and then treated with 50 ng/ml IFNg for 12 h. We isolated total RNA from cell lysates, and ICAMI, CD40 and CD86 transcripts are detected by RT-PCR analysis. Undoubtedly, ICAM-I, CD40 and CD86 mRNA levels are decreased by simvastatin in IFNg-stimulated THP-1 cells (Fig. 1C).

FIGURE 1. For dosage testing, THP-1 cells were first treated with simvastatin for 24 h at 0.1-2 μM; no cytotoxic effect was observed (data not shown). To examine its temporal effect on cell viability, cells were incubated with 2 μM simvastatin for the indicated times (A). Again, simvastatin was found to have no effect. Cell counting was performed by Cell Counting Kit-8 as described materials and methods. (B) To detect the COX-2 expression in IFNg-stimulated THP-1 cells, THP-1 cells were pre-incubated with simvastatin for 6 h and were treated with 50 ng/ml IFNg for 18 h. Protein extracts were subjected to western blotting using antibodies against COX-2 and actin (loading control). To determine relative protein expressions, a densitometry graph was drawn using the ImageJ program. (C) Cells were pre-incubated with simvastatin for 6 h and were treated with 50 ng/ml IFNg for 12 h. Then, mRNA levels were detected by RT-PCR analysis, which was performed using the primers as described in Table 1.
Simvastatin potentiates IFNg-induced COX-2 expression in RAW264.7 cells, primary peritoneal macrophages and BV2 cells
To test the effect of simvastatin on IFNg-induced COX-2 expression in murine macrophages, RAW264.7 cells were pretreated for 6 h with simvastatin and then incubated for 18 h with 50 ng/ml IFNg. Cell lysates were then western blotted to determine COX-2 protein levels. Based on previous data, we expected to find that simvastatin reduced COX-2 expression. However, IFNg-induced COX-2 expression was not inhibited by simvastatin, in fact its expression was augmented (Fig. 2A). Furthermore, we confirmed the up-regulation of COX-2 by simvastatin in IFNg-stimulated murine primary macrophages (Fig. 2B) and BV2 murine microglia cell line (Fig. 2C).

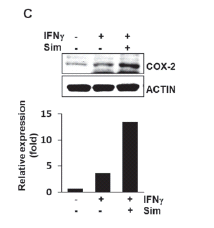
FIGURE 2. The effect of simvastatin on COX-2 expression was showed in IFNg-stimulated Raw264.7 cells (a murine macrophage cell-line) (A), primary peritoneal macrophages (B) and BV2 cells (a murine microglia cellline) (C). Cells were pre-treated with simvastatin for 6 h and then treated with 50 ng/ml IFNg for 18 h. COX-2 protein expression was detected by western blotting as described in Fig. 1; expression levels were normalized using the ImageJ program.
Simvastatin inhibits IFNg- induced STAT1/3 activation and increases SOCS1/3 transcripts in THP-1 and RAW264.7 cells
IFNg stimulation activates Jak-1 and Jak-3 and then STATs, especially STAT1 or STAT3 (Qing and Stark, 2004). IFNg-activation site (GAS) elements, which are STATs-binding DNA sites, are known to interact with phospho-STATs prior to COX-2 transcriptional activation (Decker et al., 1997). Thus, STAT1 and STAT3 are important transcription factors for IFNg-induced COX-2 expression. A previous report demonstrated that simvastatin inhibits IFNg-induced STAT1 activation in murine macrophage RAW264.7 cells (Lee et al., 2007), whereas another found that IFNg-induced STAT1 phosphorylation (activation) is not affected by simvastatin in human microvascular endothelial cells (Sadeghi et al., 2001). Thus, we examined whether simvastatin inhibits the activations of STAT1 and STAT3 in IFNg-stimulated THP-1 cells (Fig. 3A). Simvastatin was found to suppress STAT1/3 activations, indicating that simvastatin had negative effects on STAT1/3 activations in IFNg-stimulated THP-1 cells. In addition, because STAT1/3 activation has been reported to be blocked by SOCS1/3 up-regulation (Yasukawa et al., 2000), we tested whether simvastatin induces SOCS1 and SOCS3 transcripts. The result obtained showed that simvastatin up-regulates both SOCS1 and 3 mRNAs (Fig. 3A). These findings suggested that simvastatin is likely to suppress IFNg-induced COX-2 expression by blockading STAT1/3 activations by up-regulating SOCS1/3 transcription in human THP-1 cells.

FIGURE 3. THP-1 cells (A) and RAW264.7 cells (B) were pre-incubated with simvastatin for 6 h and then treated for 1 h with 50 ng/ml IFNg. Western blot analysis was performed by using antibodies against Tyr-701-phosphorylated STAT1 and Tyr-705-phosphorylated STAT3. To detect SOCS1/3 transcripts, cells were incubated for 6 h with simvastatin and then total RNA was isolated. RT-PCR was performed using the primers shown in Table 1.
Oppositely, to determine whether simvastatin activates molecules up-stream of COX-2 gene expression in RAW264.7 cells because of increased COX-2 expression, we examined its effect on IFNg-induced STAT1/3 activation. Unexpectedly, the results obtained showed that STAT1/3 activation is inhibited by simvastatin in IFNg-stimulated RAW264.7 cells, as in THP-1 cells, despite its increasing COX-2 protein levels (Fig. 3B). SOCS1/3 mRNA levels are also increased by simvastatin in RAW264.7 cells (Fig. 3B). Furthermore, we investigated whether the contrary effect of simvastatin on IFNg/STATs-induced gene expression, as well COX-2 gene expression, is detected in between THP-1 cells and RAW264.7 cells. Thus, we tested the effect of simvastatin on IFNg/STATs-induced CD86 and inducible nitric oxide synthase gene expression, known to be mediated by STATs activation. The results showed that CD86 transcripts are decreased by simvastatin in both IFNg-stimulated THP-1 (Fig. 1C) and RAW264.7 cells (Fig. 4A). However, inducible nitric oxide synthase expression was not inhibited by simvastatin in IFNg-stimulated RAW264.7 cells (Fig. 4B) and BV2 cells (data not shown), rather, was enhanced slightly. In THP-1 cells, inducible nitric oxide synthase expression was not detected by IFNg. In these data, we showed that simvastatin has a contrary effect on the expression of inflammatory molecules including COX-2, depending on their target proteins and species in inflammatory conditions.

FIGURE 4. CD86 transcripts using RTPCR (A) and inducible nitric oxide synthase (iNOS) expression level using western blot (B) were detected in RAW264.7 cells. Cells were pre-treated with simvastatin (SIM) for 6 h and then treated with 50 ng/ml IFNg for 12 h (RTPCR) or 18 h (western blot). RT-PCR was performed using the primers as described in Table 1 and western blot analysis was performed by using antibodies against inducible nitric oxide synthase antibody.
Taken together, these findings suggest that simvastatin is likely to inhibit COX-2 expression by preventing STAT1/3 activation by inducing SOCS1/3 in IFNg-stimulated THP-1 cells, but enhances COX-2 expression in IFNg-stimulated murine macrophages despite this SOCS1/3-induced STAT1/3 inactivation, furthermore, these different effects of simvastatin seem to be species and/or target proteins dependent (Fig. 5).

FIGURE 5. Proposed model of the contrary effects by simvastatin on IFNg-induced COX-2 expression in human monocytes (THP-1) and in murine macrophages (Raw264.7 cells).
Discussion
Six commercially available statins reduce serum cholesterol levels, i.e., three natural statins (simvastatin, lovastatin and pravastatin) and three synthetic statins (atorvastatin, cerivastatin and fluvastatin). Moreover, recent reports indicate that the effects of the above statins differ under inflammatory conditions (Huang et al., 2003; Habib et al., 2007; Degraeve et al., 2001; Chen et al., 2000; 2004). Furthermore, although statins have been shown to have anti-inflammatory effects, many studies have shown that statins may also have pro-inflammatory effects, for example, they may induce inducible nitric oxide synthase or pro-inflammatory cytokines (Chen et al., 2000; Kiener et al., 2001). Collectively, these results indicate that the effects of statins are dependent on statin, cell, and species types, and tissue conditions.
In the present work, we focused on the effect of simvastatin on COX-2 expression in IFNg-stimulated THP-1 and RAW264.7 cells, because IFNg-induced COX-2 expression is a known major inflammatory response. Previous studies have failed to clarify the effects of simvastatin on COX-2 expression in the inflammatory state in human and murine cells, and no previous study has demonstrated the effect of simvastatin on the IFNg/STATs/COX-2 inflammatory signaling pathway. The present study demonstrates that simvastatin suppressed IFNg-induced COX-2 expression by up-regulating SOCS1/3 mRNA expression and thus inhibiting STAT1/3 activation in IFNg-stimulated human THP-1 cells. On the other hand, in IFNg-stimulated murine RAW264.7 cells, murine primary macrophages, and BV2 cells, simvastatin was found to have no inhibitory effect on IFNg-induced COX-2 expression despite inducing SOCS1/3 mRNA and preventing STAT1/3 activation, in fact, it tended to enhance IFNg-induced COX-2 expression. Based on these current findings, we suggest that the modulatory effect of simvastatin on IFNg-induced COX-2 expression is species dependent under inflammatory conditions. However, the different mechanisms involved are beyond our speculation. We can only suppose that the different effects of simvastatin on IFNg-induced COX-2 expression are related to the balance between the strengths of the negative and positive effect of simvastatin on COX-2 expression signaling in different cellular backgrounds. In terms of the positive signaling mechanism induced by statins on COX-2 expression, a recent study demonstrated that this requires the binding of transcription factors to the C/EBPb and CRE elements on COX-2 promoter (Chen et al., 2004). Another report suggested that statins induce COX-2 expression and increase intracellular 15-deoxydelta-(12, 14)-prostaglandin J2 (15d-PGJ2) levels via ERK1/2 and p38 MAPK-dependent pathways (Yano et al., 2007). Conversely, others have shown that statins inhibit COX-2 expression via NF-κB suppression (Habib et al., 2007; Kim et al., 2007).
In addition to the effect of simvastatin on IFNg-induced COX-2 expression, it was interesting to find that STAT1/3 inactivation resulted from SOCS1/3 transcript up-regulation in both THP-1 and RAW264.7 cells. A previous study reported that lovastatin or fluvastatin induce SOCS3 gene expression in RAW264.7 cells, and that this is dependent on decreased intracellular levels of mevalonate products (Huang et al., 2003). Thus, we suggest that SOCS1/3 induction by simvastatin may also involve reductions in the levels of mevalonate pathway products such as mevalonate, farnesyl pyrophosphate and geranylgeranyl pyrophosphate.
Most importantly, we need to identify the different mechanisms by which statins affect COX-2 expression in different cells, species, and tissues, because statins are now used clinically, and because COX-2 is viewed as an important molecule in the context of cancers and inflammatory diseases. Simvastatin appears to be a potential anti-inflammatory agent and a potential treatment for COX-2 mediated-inflammatory diseases in a human system. At this stage of study, it appears that the various actions of simvastatin on COX-2 require more specific experimentation, and comparative studies on its in vivo effects on both human versus murine systems.
Acknowledgments
This study was supported by grants from the Korean Health 21 R & D Project and from the Seoul National University Hospital Research fund (03-08-072-0).
References
1. Chello M, Anselmi A, Spadaccio C, Patti G, Goffredo C, Di Sciascio G et al. (2007). Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Annals of Thoracic Surgery 83: 1374-1380. [ Links ]
2. Chen H, Ikeda U, Shimpo M, Ikeda M, Minota S, Shimada K (2000). Fluvastatin upregulates inducible nitric oxide synthase expression in cytokine-stimulated vascular smooth muscle cells. Hypertension 36: 923-928. [ Links ]
3. Chen JC, Huang KC, Wingerd B, Wu WT, Lin WW (2004). HMGCoA reductase inhibitors induce COX-2 gene expression in murine macrophages: role of MAPK cascades and promoter elements for CREB and C/EBPbeta. Experimental Cell Research 301: 305-319. [ Links ]
4. Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC et al. (2007). Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Science 81: 288-98. [ Links ]
5. Chung HK, Lee IK, Kang H, Suh JM, Kim H, Park KC, Kim DW, Kim YK, Ro HK, Shong M (2002). Statin inhibits interferongamma-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Experimental and Molecular Medicine 34: 451-461. [ Links ]
6. Decker T, Kovarik P, Meinke A (1997). GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. Journal of Interferon and Cytokine Research 17: 121-134. [ Links ]
7. Degraeve F, Bolla M, Blaie S, Creminon C, Quere I, Boquet P et al. (2001). Modulation of COX-2 expression by statins in human aortic smooth muscle cells. Involvement of geranylgeranylated proteins. Journal of Biological Chemistry 276: 46849-46855. [ Links ]
8. Ghittoni R, Napolitani G, Benati D, Ulivieri C, Patrussi L, Laghi Pasini F et al. (2006). Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. European Journal of Immunology 36: 2885-2893. [ Links ]
9. Greenwood J, Mason JC (2007). Statins and the vascular endothelial inflammatory response. Trends in Immunology 28: 88-98. [ Links ]
10. Grip O, Janciauskiene S, Lindgren S (2000). Pravastatin down-regulates inflammatory mediators in human monocytes in vitro. European Journal of Pharmacology 410: 83-92. [ Links ]
11. Habib A, Shamseddeen I, Nasrallah MS, Antoun TA, Nemer G, Bertoglio J, Badreddine R, Badr KF (2007). Modulation of COX-2 expression by statins in human monocytic cells. FASEB Journal 21:1665-1674. [ Links ]
12. Huang KC, Chen CW, Chen JC, Lin WW (2003). HMG-CoA reductase inhibitors inhibit inducible nitric oxide synthase gene expression in macrophages. Journal of Biomedical Science 10: 396-405. [ Links ]
13. Huang KC, Chen CW, Chen JC, Lin WW (2003). Statins induce suppressor of cytokine signaling-3 in macrophages. FEBS Letter 555: 385-389. [ Links ]
14. Inoue H, Tanabe T (1998). Transcriptional role of the nuclear factor kappa B site in the induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochemical and Biophysical Research Communications 244: 143-148. [ Links ]
15. Kiener PA, Davis PM, Murray JL, Youssef S, Rankin BM, Kowala M (2001). Stimulation of inflammatory responses in vitro and in vivo by lipophilic HMG-CoA reductase inhibitors. International Immunopharmacology 1: 105-118. [ Links ]
16. Kim YS, Ahn Y, Hong MH, Kim KH, Park HW, Hong YJ et al. (2007). Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. Journal of Cardiovascular Pharmacology 49: 376-383. [ Links ]
17. Kobashigawa JA (2004). Statins and cardiac allograft vasculopathy after heart transplantation. Seminars in Vascular Medicine 4: 401-406. [ Links ]
18. Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ (2005). Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Human Immunology 66: 653-665. [ Links ]
19. Lee SJ, Qin H, Benveniste EN (2007). Simvastatin inhibits IFNgamma-induced CD40 gene expression by suppressing STAT-1alpha. Journal of Leukocyte Biology 82: 436-447. [ Links ]
20. Liao JK (2004). Statins: potent vascular anti-inflammatory agents. International Journal of Clinical Practice. 48 Supplement 143: 41-48. [ Links ]
21. Miller LJ, Chacko R (2004). The role of cholesterol and statins in Alzheimer's disease. Annals of Pharmacotherapy 38: 91-98. [ Links ]
22. Monick MM, Powers LS, Butler NS, Hunninghake GW (2003). Inhibition of Rho family GTPases results in increased TNFalpha production after lipopolysaccharide exposure. Journal of Immunology 171: 2625-2630. [ Links ]
23. Ortego M, Bustos C, Hernandez-Presa MA, Tunon J, Diaz C, Hernandez G et al (1999). Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis 147: 253-261. [ Links ]
24. Qing Y, Stark GR (2004). Alternative activation of STAT1 and STAT3 in response to interferon-gamma. Journal of Biological Chemistry 279: 41679-41685. [ Links ]
25. Sadeghi MM, Tiglio A, Sadigh K, O'Donnell L, Collinge M, Pardi R et al (2001). Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation 71: 1262-1268. [ Links ]
26. Tunon J, Martin-Ventura JL, Blanco-Colio LM, Egido J (2007). Mechanisms of action of statins in stroke. Expert Opinion on Therapeutic Targets 11: 273-278. [ Links ]
27. Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R (2003). Mechanism of cyclooxygenase-2 upregulation in late preconditioning. Journal of Molecular and Cellular Cardiology 35: 525-537. [ Links ]
28. Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K et al (2007). Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circulation Research 100: 1442-1451. [ Links ]
29. Yasukawa H, Sasaki A, Yoshimura A (2000). Negative regulation of cytokine signaling pathways. Annual Review of Immunology 18: 143-164. [ Links ]
Received: December 2, 2008.
Accepted: February 17, 2009.














