Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.33 n.2 Mendoza mayo/ago. 2009
BRIEF NOTE
Differences in intracellular localization of corn stunt spiroplasmas in magnesium treated maize.
Claudia Nome1*, Paulo César Magalhães2, Elizabeth Oliveira2, Sergio Nome and Irma Graciela Laguna1
1. Instituto Nacional de Investigaciones Agropecuarias - Instituto de Fitopatología y Fisiología Vegetal (INTA-IFFIVE). Córdoba. Argentina
2. Embrapa Milho e Sorgo. Caixa Postal 151 - 35701-970, Sete Lagoas, MG, Brasil.
*Address correspondence to: Claudia Nome. Instituto Nacional de Investigaciones Agropecuarias - Instituto de Fitopatología y Fisiología Vegetal (INTA-IFFIVE). Camino 60 cuadras Km 5 1/2. X5020ICA Córdoba, ARGENTINA. E-mail: cfnome@yahoo.com.ar
ABSTRACT: Maize plants infected with Spiroplasma kunkelii show symptoms similar to that of plants in a magnesium-deficient soil, and it has been shown that the spiroplasma alters the plants' magnesium absorption. In the current study we compared changes associated to either spiroplasma infection, two soil magnesium levels and their combinations. Plant symptoms were recorded and correlated with transmission electron microscopy observations. Plants grown on a high magnesium treatment showed no macroscopical alterations nor organelle ultrastructural alterations, while plants on a low magnesium treatment showed macroscopical vein yellowing and, ultrastructurally, they had most chloroplasts and mitochondrial membranes altered. Infected plants on a low magnesium treatment had an ageing aspect, ultrastructurally showed chloroplasts and mitochondrial alterations similar to those non-infected and grown on a low magnesium treatment, and spiroplasma cells were found in phloem cells, but outside their cytoplasm. Infected plants on a high magnesium treatment showed similar symptoms and ultrastructural alterations as either non-infected plants on the low magnesium treatment or in infected plants on the low magnesium treatment, but differ from them in that the spiroplasma cells were located inside the cytoplasm. Results suggest that magnesium is involved in the plant-pathogen interaction.
Key words: Mollicutes; Cytopathology; Plant pathogen; Magnesium deficiency
Plant pathogens include viruses, viroids, fungi, bacteria, and within the latter, the Mollicutes, a bacterial class including the families Mycoplasmataceae, Spiroplasmataceae and Acheloplasmataceae. They are the smallest known prokaryotes and they lack a cell wall. Their genome oscillates from 577 to 2220 kpb, and they are extremely demanding from the nutritional point of view, therefore very difficult to grow in culture medium (Bové, 1988).
The Spiroplasmataceae include the genus Spiroplasma, whose name derives from the helical morphology and the motility of cells in liquid media. They have been recognized in diseased plants less than thirty years ago, and they have been recognized also as pathogens of warm-blooded animals and insects (Maramorosch, 1981; Bastian et al., 2007). They are diagnosed by serological and molecular methods, and by electron microscopy.
The genus name Phytoplasma has not yet been formally recognized, but it is currently at "candidatus status" and is used for [Spiroplasmataceae] bacteria that cannot be cultured, and they belong to the monophyletic order Acholeplasmatales (Lee et al., 2000). They are only detected by molecular methods, electron microscopy, and by the response of the host plant to antibiotic treatment. Phytoplasmas exist both in plants and in insects feeding on phloem of infected plants. As for spiroplasmas, such insects have been described as the pathogen vectors. Inside the plants, both phytoplasmas and spiroplasmas are confined out of the cytoplasm of the sieve cells, while in insects they are distributed in several tissues (Conci et al., 2001; Ammar and Hogenhout, 2005).
Spiroplasma kunkelii is a maize pathogen transmitted by the leafhopper Dalbulus maydis (A+Y), producing a disease named corn stunt disease. It has been reported that the infected plants show a lower magnesium absorption, Oliveira et al. (2002, 2005).
The symptoms of maize plants grown on magnesium deficient soil (concentrations below 0,5 Cmol c dm-3) are leaf veins yellowing/ whitening all along their length, and purple coloring on the tips of older leaves, Coelho and França (1995). Almost identical symptoms are the ones produced by the spiroplasma infection (Tsai and Miller, 1995).
The aim of this work was to compare plant alterations associated with low soil magnesium levels and by the infection with S. kunkelii, and to assess the possibility of spiroplasma-induced alterations were reverted by a high magnesium availability.
Zea mays plants, cv. Pop Zélia, were breed on soil from the Brazilian Cerrado (savanna) with naturally shows low magnesium levels, to which magnesium was added to obtain either 0.20 or 1.0 Cmol c dm-3 (they will be referred to as the low and the high magnesium treatments). They were kept in greenhouse with stable conditions of 27ºC, 16 hours of lighting and 60% of humidity. Some plants were kept uninfected, while others were exposed to the leafhopper Dalbulus maydis for inoculation with S. kunkelii after seven days of plant emergence.
Sheath tissue samples of maize leaves (2 x 3 mm) sixty seven days old (sixty days after inoculation) were collected and fixed in Karnovsky fluid, post fixed in osmium tetroxide 1%, contrasted in uranyl acetate 0,5%, dehydrated in acetone solution series, pre-embedded in acetone-epoxy resin, and embedded in low viscosity Spurr resin (polymerized at 70ºC for 2 days). Ultrathin sections were made with a diamond blade and sections were contrasted with lead citrate pH 12 and with uranyl acetate 2%. Sections were observed with a transmission electron microscope Jeol 1220 JEM EXII.
Uninfected plants on the high magnesium treatment showed no abnormal symptoms, while maize plants on the low magnesium treatment showed vein yellowing all along their leaves and some whitening down on the sheath. Also, infected plants cultivated on low magnesium treated soil appeared old, but showed a more intense purple coloring and sometimes even leaf apex necrosis. Infected plants on the high magnesium treatment also showed vein yellowing all along the sheath and purple coloring on older leaves; such plants appeared old, though they were less damaged than spiroplasma infected plants on the low magnesium treatment.
As expected, uninfected plants on high magnesium treatment showed no ultrastructural alterations (Fig. 1). However, uninfected plants on the low magnesium treatment exhibited chloroplast alterations, swollen chloroplasts with disordered grana, in all of the studied tissues (Fig. 2). Spiroplasma infected plants on the low magnesium treatment also showed disorganized chloroplasts, mainly in their veins, and the pathogen was seen inside phloem sieve tubes, but not in the cytoplasm (Figs. 3, 5). In contrast, infected plants on the high magnesium treatment, showed the spiroplasma both outside and inside the cytoplasm of phloem cells (Figs. 4, 6).
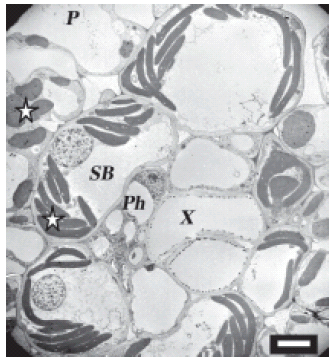
FIGURE 1. Uninfected maize cells on the high magnesium treatment. Normal chloroplasts are seen in the sheath bundle and the parenchyma. Vascular bundle shows no spiroplasmas. In this and in all subsequent figures, stars indicate chloroplasts; arrows indicate spiroplasmas; Cy is cytoplasm; CW, cell wall; SB, sieve bundle; X, xylem; Ph, phloem; P, phloem parenchyma. Scale bar represents 7 μm.

FIGURE 2. Uninfected maize cells on the low magnesium treatment.. Picture shows distorted chloroplasts of sheath bundle and normal parenchymal chloroplasts. Scale bar represents 2 μm.
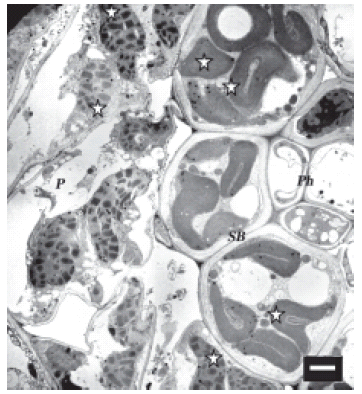
FIGURE 3. Spiroplasma infected maize cells on the low magnesium treatment. Micrograph shows devastated chloroplasts both in sheath bundle and the parenchyma. Scale bar represents 2 μm.

FIGURE 4. Sieve tube of infected maize plant on the low magnesium treatment with spiroplasmas. Ordinary image of spiroplasmas spread loose out of the cytoplasm. Scale bar represents 500 nm.
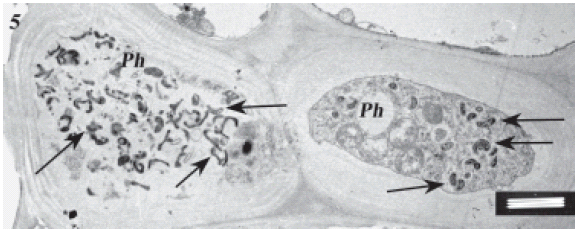
FIGURE 5. Sieve tube of an infected maize plant on the high magnesium treatment showing spiroplasmas within their cells. Notably, the pathogen is not only within sieve tubes but also inside cytoplasm of young phloem cells and phloem parenchyma. Scale bar represents 1 μm.

FIGURE 6. Same as in Fig. 5. Scale bar represents 1 μm.
It is known that low magnesium concentrations induce maize plants to show leaf vein yellowing (INFOPOS, 2001; Bull, 1993). Earlier studies have shown that S. kunkelii infection reduces magnesium concentration in maize plants (Oliveira et al., 2002, 2005; Ammar et al., 2005). We may suggest, therefore, that the symptom expression may be related to a competition for the cation between the plant and the pathogen.
Infected plants on the low magnesium treatment were more damaged than infected plants on the high magnesium treatment. It is notable, however, that the spiroplasma locates inside of the young phloem cells of infected plants on the high magnesium treatment. This may suggest that magnesium influences the way that the pathogen invades the plant, and that it allows the spiroplasma to introduce inside the young cells, and to remain alive, and to proliferate within their cytoplasm.
References
1. Ammar E, Hogenhouta S (2005). Use of Immunofluorescence Confocal Laser Scanning Microscopy to Study Distribution of the Bacterium Corn Stunt Spiroplasma in Vector Leafhoppers (Hemiptera: Cicadellidae) and in Host Plants. Annals of the Entomological Society of America 98: 820-826. [ Links ]
2. Bastian F, Sanders D, Forbes W, Hagius S, Walker J, Henk W, Enright FM, Elzer PH (2007). Spiroplasma spp. from transmissible spongiform encephalopathy brains or ticks induce spongiform encephalopathy in ruminants. Journal of Medical Microbiology 56: 1235-1242. [ Links ]
3. Bull L (1993). Nutrição mineral do milho. In: Simposio sobre fatores que afetam a produtividade do milho e do sorgo. In: Bull, L.T.; Cantarella, H. (Eds.).Cultura do milho; fatores que afetam a produtividade. Piracicaba: POTAFOS, p. 63-145. [ Links ]
4. Bové J (1988). Plant mollicutes: phloem-restricted agents and surface contaminants. Acta Horticulturae 225: 215-222. [ Links ]
5. Coelho A, França G (1995). Nutrição e Adubação. 2 ed. Aum. In: Arquivo Agronômico, n.º 2, POTAFÓS. (Piracicaba, SP). Seja o doutor de seu milho. Piracicaba: p. 1-9. [ Links ]
6. Conci L, Torres L, Galdeano E, Meneguzzi N, Nome C, Docampo D, Nome S, Paradell S, Conci VC, Ramallo JC (2001). Fitoplasmas. Caracterización molecular, diagnóstico y epidemiología de fitoplasmas que afectan cultivos de importancia económica. In: Memoria anual INTA-IFFIVE. INTA. Buenos Aires, Argentina, pp. 103. [ Links ]
7. INFOPOS (2001). Conozca y Resuelva los Problemas del Maíz. International Plant Nutrition Institute. Tríptico SP-0801. [ Links ]
8. Lee I, Davis R, Gundersen-Rindal D (2000). Phytoplasma: Phytopathogenic Mollicutes. Annual Review of Microbiology 54: 221-55. [ Links ]
9. Malavota E, Haang H, de Mello F, Brasil Sobr M (1964). Los elementos nutritivos escenciales. In: La nutrición mineral de algunas cosechas tropicales. Malavota E, Haang HP, de Mello FAF and Brasil Sobr MOC. Eds. Instituto Nacional de la potasa Bernal, Suiza, pp. 24-25. [ Links ]
10. Maramorosch K (1981). Spiroplasmas: Agents of Animal and Plant Diseases. BioScience 31: 374-380. [ Links ]
11. Oliveira C, Cruz I, Schaffert R (2002). Growth and nutrition of mollicute infected maize. Plant Disease 86: 945-949. [ Links ]
12. Oliveira E, Oliveira C, Souza I, Magalhães P, Andrade C, Hogenhout S (2005). Spiroplasma and phytoplasma reduce kernel production and nutrient and water contents of several but not all maize cultivars. Maydica 50: 171-178. [ Links ]
13. Tsai J, Miller J (1995). Corn Stunt Spiroplasma. Plant Pathology Circular 373. [ Links ]
Received: May 23, 2008.
Revised version received: June 1, 2009.
Accepted: June 2, 2009.














