Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell v.33 n.3 Mendoza sept./dic. 2009
ORIGINAL ARTICLES
Changes in the corpora allata and epidermal proliferation along the fourth instar of the Chagas disease vector Triatoma infestans
Jorge R. Ronderos
Centro Regional de Estudios Genómicos (CREG-UNLP) and Cátedra de Histología y Embriología Animal (FCNyM-UNLP) La Plata, ARGENTINA.
Address correspondence to: Jorge R. Ronderos. E-mail: jrondero@museo.fcnym.unlp.edu.ar
ABSTRACT: Triatoma infestans, a blood-feeding insect, synchronises physiological mechanisms leading to moult with food intake. Since the corpora allata are important in moult and metamorphosis regulation, we have studied morphological changes in 4th instar nymphs (gland size, cell density, percent of animals showing mitoses and cell size). Changes were correlated with the effect of precocene II, epidermal proliferation, and with the extent of the "head critical period". Based on morphological grounds, three stages can be defined in the gland along the 4th instar: Stage 1 (days 0-2 after feeding) showed small corpora allata, composed by a small number of cells, and in which mitoses were absent; Stage 2 (days 3-9) showed growing corpora allata, in which cell number was increasing and proliferation was apparent; and Stage 3 (days 10-13) showed no mitotic activity, and a sharply diminishing size of the gland, as a consequence of the diminishing size of their cells. The ability of precocene II to induce abnormal moulting disappeared during stage 2 correlating with the termination of the head critical period and suggesting that corpora allata are essential during days 3 to 5 to determine normal growth. Epidermal cell number was increasing as a consequence of more frequent mitotic activity, beginning after the finalization of the head critical period and after a first increment in the size of the gland.
Key words: Moult; Neuroendocrine control; Juvenile hormones; Precocene II
Introduction
The Chagas disease vector Triatoma infestans (Klug 1834) (Hemiptera, Reduviidae) is a blood-feeding insect in which physiological mechanisms leading to moult are synchronised with food intake. Once blood intake is accomplished, neurosecretions from the protocerebrum act on the corpora allata and on the prothoracic glands stimulating the secretion of both juvenile hormones and ecdysteroids (Steel et al., 1982). Co-ordinated activity of these hormones regulates growth and metamorphosis (Dubrovsky, 2005), involving proliferation and differentiation on target tissues.
Except for the work of Baehr et al. (1978), information regarding variations of juvenile hormones' circulating levels in triatomine insects is scarce. Several authors have proposed that morphological variations of the corpora allata correlate well with juvenile hormones synthetic activity (Szibbo and Tobe, 1981; Sedlak, 1983; Tobe et al., 1984; Johnson et al., 1993; Chang et al., 2005). Therefore, we studied morphological changes in the corpora allata (organ size, cell number and size, and mitotic activity) and correlated them with the extent of the "head critical period" (Wigglesworth, 1934), the ability of precocene II to induce abnormal moulting, and changes in cell number and mitotic activity in the abdominal epidermis along the 4th instar of T. infestans.
Materials and Methods
Insects
Fourth-instar Triatoma infestans nymphs were obtained from an artificial colony maintained at 28ºC and 40% relative humidity, under a 12:12 hours light-dark period. Insects reaching the 4th instar were isolated and starved during 21 days before an ad libitum blood meal (from chicken) was offered. Under these conditions, the duration of the 4th instar was 13±1 days, reaching apolysis on day 10. Groups of 4-8 insects were sacrificed immediately before (non-fed insects, Day 0) or on different days after the blood meal.
Morphological analysis of the corpora allata
Corpora allata, together with the brain, the corpora cardiaca and the surrounding organs, were dissected and fixed in Bouin solution during 12 hours, dehydrated and embedded in paraffin for histological analysis.
Every brain-corpora cardiaca-corpora allata complex was cut serially (3 μm thick) along its longitudinal axis and stained with hematoxylin and eosin. The size of the corpora allata was estimated based on the area of the largest section obtained, which was amplified by the use of a camera lucida. The area was calculated by the Simpson's integrated area methodology: briefly, a number of intervals were traced along the main axis of the surface, and the distances between borders for each interval were recorded. The area was calculated as:
Area = I/3 + (4O + 2P + E)
where I: value of the interval; O: summation of odd intervals; P: summation of the pairs intervals and E: extreme value.
The number of cells in the gland was estimated as the number of cells contained in each largest section. The relative changes in the gland's cell size were also estimated as the mean number of cells contained in an arbitrary area of 1 mm2. So, increases in the number of cells/mm2 reflected a minor mean cell size, while decreases in this relation reflected an increase in mean cell size. The occurrence of mitoses (or not) was also recorded in each studied gland.
Effects of precocene II
One hundred μg of precocene II (6,7-dimethoxy-2,2-dimethylchromene, Sigma Chemical Company), an inhibitor of juvenile hormone production, diluted in 5 μl of acetone, were topically administered as a single dose, either on the day before feeding or on days 1-7 after blood intake. A group of 15 insects was treated topically with acetone as control. After moult, morphological alterations in the resulting adultoids were observed and the percentage of insects reaching abnormal moults on each day of treatment was recorded. Finally, adultoids were dissected to observe gonadal development.
Determination of the head critical period
Groups of insects were decapitated at different times before and after blood intake to determine the head critical period (Wigglesworth, 1934). After decapitation, the prothorax was sealed with wax. Insects were sacrificed on day 10 (day of apolysis) and the percentage of insects effectively reaching apolysis was recorded.
Epidermal growth and proliferation
To evaluate epidermal growth, the dorsal part of the abdominal epidermis of insects sacrificed between days 1 and 9 after blood intake were dissected and whole mounted after staining with hematoxylin and eosin. The total number of cells in a microscopic field (1000x) was recorded for each insect. The percentage of insects presenting proliferating epidermis (i.e. showing mitotic figures) was also recorded in the same experiment.
Results
Morphological changes in the corpora allata
Corpora allata, corpora cardiaca, and surrounding organs are shown in figure 1a.
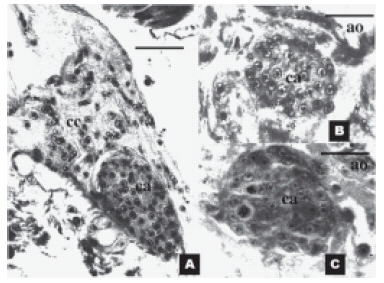
FIGURE 1. Histological sections of the corpora allata and
surrounding organs of 4th instar nymphs of Triatoma infestans,
at different times after blood intake. (A) Longitudinal section
through the corpora allata, on day 8 after blood intake, showing
its anatomical relation with the corpora cardiaca. Scale
bar represents 50 μm (B) Transverse section through the
corpora allata, 1 day after blood intake and (C) 6 days after
blood intake showing two mitotic figures (1000x). Scale bars
in B and C represent 25 μm. ca: corpora allata; cc: corpora
cardiaca; ao: aorta.
The size of the gland increased after blood intake, reaching two peaks, the first one on day 4 and the second one on day 8. Then, the size decreased slowly until ecdysis occurred Fig. 2a). Cell number also increased after blood intake, reaching a peak between days 11 and 12 (Fig. 2b). The percent of animals showing proliferating glands was restricted to a period ranging from days 3 to 9 (reaching a 100% peak on day 5; Fig. 2c). The cell size reached a maximum on day 4 and decreased slowly thereafter, reaching minimal values towards the end of the cycle (Fig. 2d).

FIGURE 2. Morphological changes in the corpora allata of
4th instar nymphs of Triatoma infestans. (A) Gland size (surface
of largest section in μm2). (B) Gland cell number (estimated
as the number of cells in the largest section obtained).
(C) Percent of nymphal glands showing mitoses. (D) Cell size
(estimated through the number of cells/mm2 relation). Each
point represents mean ± SE of 4-7 cases.
Effects of precocene II
Single dose administration of precocene II, either on day 0 (before feeding), or on each of days 1 to 7 after feeding, resulted in different percentages of nymphs with morphological alterations (adultoids) after moulting. Adultoids retained some juvenile characters while acquired several morphological characters of the imago such as an adult-like thorax, connexives and ocelli. The wings were present but they were not properly developed. None of the adultoids were able to feed, and most of them died after a few days or during ecdysis. Genitalia were developed but the insects were sexually immature. On the contrary, all control insects showed normal ecdysis to 5th instar nymphs.
The percent of adultoids obtained after each treatment is presented in figure 3a. All treated nymphs on days 0, 1 and 2 after blood meal resulted in adultoids. After this, the number of adultoids decreased, and precocene II treatment was totally ineffective when administered on days 6-7 after blood intake.

FIGURE 3. (a) Percent of adultoids obtained after treatment
of Triatoma infestans 4th instar nymphs with a single
topical dose of 100 μg of precocene II, on different days
before (day 0) and after blood meal (days 1-7). (b) Extension
of the head critical period as shown by the percent of
nymphs reaching apolysis on day 10 after blood feeding.
Head critical period
Head ablation performed on days 0 to 4 was followed by inhibition of apolysis in all insects treated. The percent of animals displaying apolysis increased steeply from 50 to 100% when the head was ablated on days 5-9 after the meal (Fig. 3b).
Epidermal proliferation
Proliferation in epidermis (that precede and prepare for apolysis and moulting) were studied by determining the percent of animals showing epidermal mitoses (Fig. 4a) and by evaluation of the epidermal cell number (Fig. 4b). Mitotic activity was restricted to a period between days 4 and 8 after blood intake. Cell number increased steadily, reaching peak values on day 9.

FIGURE 4. Changes in the abdominal epidermis of Triatoma
infestans 4th instar nymphs during the first nine days of the
moulting cycle. (a) Percent of nymphs showing epidermal
mitoses. (b) Cell density (number of cells per optical field)
(1000x). Each point represents mean ± SE of 4-7 cases.
Discussion
On the basis of the morphological changes observed in the current study, three stages can be defined in the corpora allata along the moulting cycle in 4th instar Triatoma infestans (Figs. 1 and 2): Stage 1 (days 0-2 after feeding), in which the corpora allata were small, cells were large, but their cell number was low and mitoses were absent (Fig. 1b); Stage 2 (days 3-9), in which the corpora allata were growing, the number of cells was increasing and proliferation was occurring to different extents (Fig. 1c); and Stage 3 (days 10-13), in which the size of the corpora allata diminished sharply, as a consequence of the diminishing size of their cells, and in which it was no mitotic activity. During Stage 2, the ability of precocene II to induce abnormal moulting disappeared, coinciding with the finalization of the head critical period and the first increment of the size of the corpora allata (Figs. 2 and 3). Concomitantly to corpora allata stage 2, epidermal cell number was increasing as a consequence of more frequent mitoses (Fig. 4).
The existence of correlative changes in the morphology of the corpora allata and the synthesis and circulating titers of juvenile hormones has been postulated for several insect species (Wigglesworth, 1934, 1936, 1948; Scharrer, 1971, 1978; Lanzrein, 1978; Szibbo and Tobe, 1981; Sedlack, 1983; Tobe et al., 1984). Our results suggest that corpora allata develops the highest activity during Stage 2, (i.e. between days 3-9 after blood intake).
The analysis of changes in the size of the corpora allata also suggests that the gland develops its activity mainly during stage 2 (reaching two peaks on days 4 and 8 after blood intake). Both peaks are preceded by an increase of the number of cells of the gland.
Szibbo and Tobe (1981) and Tobe et al. (1984) referred that, increments in the total number of cells in the corpora allata correlates with higher levels of both synthesis and circulating titers of juvenile hormones. The preceding high proliferation in the gland could be a condition to augment the synthetic machinery to produce great quantities of juvenile hormones (Tobe et al., 1984). Baehr et al. (1978) showed the existence of two peaks of juvenile hormones, as well as high titers of the hormone between both peaks during the 4th instar of another triatomine species. Accordingly, both gland size and cell density reached its height during the Stage 2; besides that, a transient increase occurred in both gland size and cell density on day 4 after feeding during the 4th instar of T. infestans (Figs. 2a and 2b).
From day 9 and up to moulting on day 13 the number of cells per gland was still increasing, reaching a maximum between days 10-12, but the size of the cells and the size of the gland decreased, suggesting that the gland is developing a low activity during Stage 3. However, the increment in the number of cells might be advantageous after metamorphosis, when juvenile hormones will be actively involved in reproductive processes.
Precocene II is a plant-derived compound which induces corpora allata atrophy and leads to abnormal moults in several insect species (Bowers and Martinez-Pardo, 1977; Pratt and Bowers, 1977; Unnithan et al., 1977; Liechty and Sedlak, 1978; Pener et al., 1978; Schooneveld, 1979). Since it may induce atrophy of the corpora allata, it has been used to provoke a kind of chemical allatectomy. In this way, the importance of juvenile hormones on different physiological processes as muscle maturation (Rose et al., 2001), dealation in ants (Burns et al., 2002), control of wing development (Bertuso et al., 2002) or fatty acids metabolism (Chen et al., 2005) have been studied. As it would be expected, its effects are reverted by juvenile hormone treatment (Garcia et al., 1987).
A single topical dose (100 µg) of precocene II led to abnormal moults in all cases treated between days 0-2 after blood intake, and became completely ineffective after day 5. It seems therefore, that precocene II is mainly effective during stage 1. As this compound was only partially effective during days 3-5, coinciding with the first peak of the size of the corpora allata, it may be hypothesised that gland activity during these days could be critical for the genetic reprogramming of target tissues like the epidermis.
The activity of the corpora allata is supposed to be regulated by signals coming from the brain (Wiggesworth, 1934). The neuropeptide allatotropin, isolated on the basis of its ability to stimulate the synthesis of juvenile hormones, has been found and characterised in several insect species (Kataoka et al., 1989; Veenstra and Costes, 1999; Truesdell et al., 2000; Lee et al., 2002; Park et al., 2002; Abdel-latief et al., 2003). We have recently shown the presence of an allatotropin-like peptide in the Malpighian tubules of T. infestans, establishing for the first time the endocrine function of the renal tubules in insects and the presence of this peptide also in the digestive system (Santini and Ronderos, 2007; Santini and Ronderos, 2009 a, b).
Preliminary results suggest the presence of axonal fibres which express an allatotropin-like peptide and reach the corpora allata of T. infestans (unpublished results). The presence of allatotropin-like immunoreactivity in the corpora allata of the fourth-instar T. infestans suggests that this peptide could be regulating the synthesis of juvenile hormones in this species too.
The current study showed that the head critical period finishes after day 5 (i.e. during the last part of Stage 2) in T. Infestans. This may be related with the activity of neuropeptides as prothoracicotropic hormone (PTTH) which might be stimulating ecdysteroid synthesis and release by the prothoracic glands. Indeed, the corpora allata may also participate in the regulation of ecdysteroids secretion: in fact, the corpora allata of Manduca sexta are a site of release of PTTH (Agui et al., 1980) and it has been proposed that ecdysteroids secretion depends on juvenile hormones production by the corpora allata in Rhodnius prolixus (Garcia et al., 1987).
Baehr and co-workers (1978) also showed that ecdysteroids titers in R. prolixus hemolymph increase while juvenile hormones activity is high, finding the second peak of juvenile hormones just after the pea k of ecdysteroids in hemolymph. Also, Furtado et al. (1976) have shown in Panstrongylus megistus that the highest ecdysteroids titers were found on day 11 of the 4th instar (the total length of this phase was 19 days in their study), i.e., both species show their highest ecdyteroids titers around 50-60% of the total length of the cycle. It may be hypothesised that ecdysteroids levels were highest around days 7-8 in T. infestans.
We have also studied the epidermal changes which are correlative to the above mentioned observations (morphological indications of corpora allata activity, effects of precocene II and extension of the head critical period). Though ecdysteroid receptor expression in R. prolixus is likely to occur within a few hours after feeding (Vafopoulou et al., 2005), changes in epidermal cell density in T. infestans were only evident during the last part of Stage 2, when it reached values which were approximately twice the baseline. These changes were preceded by the beginning of the mitotic activity on day 4, and which ended on day 9, when the maximum cell density previous to apolysis was observed.
The current study has shown correlative changes at four levels of the 4th instar control system in T. infestans: (1) the protocerebrum (through determining the head critical period), (2) the corpora allata (through the study of morphological changes that can be correlated with endocrine activity, as well as with (3) the effects of an allatostatic compound and (4) the growth of the abdominal epidermis. Altogether they show that a wave of cell proliferation and growth in the corpora allata occurs under protocerebral control, and goes through three distinct stages in the gland.
Finally, changes in the morphology of the corpora allata strongly suggest variations in the synthetic activity of the gland. The analysis of growth using microscopical methods could still result in a simple method to analyse the action of allatotropic and allatostatic peptides acting on the regulation of the corpora allata in T. infestans.
References
1. Abdel-Latief M, Meyering-Vos M, Hoffmann KH (2003). Molecular characterisation of cDNAs from the fall armyworm Spodoptera frugiperda encoding Manduca sexta allatotropin and allatostatin preprohormone peptides. Insect Biochemistry and Molecular Biology 33: 467-476. [ Links ]
2. Agui N, Bollenbacher WE, Granger NA, Gilbert LI (1980). Corpus allatum is release site for insect prothoracicotropic hormone. Nature 285: 669-670. [ Links ]
3. Baehr JC, Porcheron P, Dray F (1978). Endocrinologie des invertebrés. Dosages radio-immunologiques des ecdysteroides et des hormones juveniles au cours des derniers stades larvaires de Rhodnius prolixus. Comptes rendus de l'Académie des Sciences, Paris 287 D: 523-526. [ Links ]
4. Bertuso AG, Morooka S, Tojo S (2002). Sensitive periods for wing development and precocious metamorphosis after precocene treatment of the brown planthopper, Nilaparvata lugens. Journal of Insect Physiology 48: 221-229. [ Links ]
5. Bowers WS, Martinez-Pardo R (1977). Antiallatotropins: Inhibition of corpus allatum development. Science 179: 1369-1371. [ Links ]
6. Burns SN, Teal Pea, Vander Meer RK, Nation JL, Vogt JT (2002). Identification and action of JHIII from sexually mature alate females of the red imported fire ant, Solenopsis invicta Journal of Insect Physiology 48: 357-365. [ Links ]
7. Chang L-W, Tsai C-M, Yang D-M, Chiang A-S (2005). Cell size control by ovarian factors regulates juvenile hormone synthesis in corpora allata of the cockroach, Diploptera punctata. Insect Biochemistry and Molecular Biology 35: 41-50. [ Links ]
8. Chen Z, Madden RD, Dilwith JW (2005). Effect of precocene II on fatty acid metabolism in the pea aphid, Acyrthociphon pisum, under cold stress. Journal of Insect Physiology 51: 411-416. [ Links ]
9. Dubrovsky EB (2005). Hormonal cross talk in insect development. Trends in Endocrinology and Metabolism 16: 6-11. [ Links ]
10. Furtado AF, PorcheroN P, Dray F (1976). Evolution du taux des ecdysones au cours des deux derniers intermues de Pastrongylus megistus (Heteroptera: Reduviidae). Comptes rendus de l'Académie des Sciences Paris 283 D: 1077-1080. [ Links ]
11. Garcia ES, Furtado AF, Azambuja P (1987). Effect of allatectomy on ecdysteroid dependent development of Rhodnius prolixus larvae. Journal of Insect Physiology 33: 729-732. [ Links ]
12. Johnson GD, Stay B, Chan KK (1993). Structure-activity relationship in corpora allata of the cockroach Diploptera punctata: roles of mating and the ovary. Cell and Tissue Research 274: 279-293. [ Links ]
13. Kataoka H, Toschi A, Li JP, Carney RL, Schooley DA, Kramer SJ (1989). Identification of an Allatotropin from adult Manduca sexta. Science 243: 1481-1483. [ Links ]
14. Liechty L, Sedlak BJ (1978). The ultrastructure of precocene-induced effects on the corpora allata of the adult milkweed bug Oncopeltus fasciatus. General and Comparative Endocrinology 36: 433-436. [ Links ]
15. Lanzrein B (1978). Correlation between hemolymph Juvenile Hormone titer, corpora allata volume, and corpora allata in vivo and in vitro activity during oocyte maturation in a cockroach (Nauphoeta cinerea). General and Comparative Endocrinology 36: 339-345. [ Links ]
16. Lee K-Y, Chamberlin ME, Horodisky FM (2002). Biological activity of Manduca sexta allatotropin-like peptides, predicted products of tissue-specific and developmentally regulated alternatively spliced mRNAs. Peptides 23: 1933-1941. [ Links ]
17. Park C, Hwang J, Kang S, Lee B (2002). Molecular characterization of a cDNA from the silk moth Bombyx mori encoding Manduca sexta Allatotropin peptide. Zoological Science 19: 287-292. [ Links ]
18. Pener MP, Orshan L, De Wilde J (1978). Precocene II causes atrophy of corpora allata in Locusta migratoria. Nature 272: 350-353. [ Links ]
19. Pratt GE, Bowers WS (1977). Precocene II inhibits juvenile hormone biosynthesis by cockroach corpora allata in vitro. Nature 265: 548-550. [ Links ]
20. Rose U, Ferber M, Hustert R (2001). Maturation of muscle properties and its hormonal control in an adult insect. Journal of Experimental Biology 204: 3531-3545. [ Links ]
21. Santini MS, Ronderos JR (2007). Allatotropin-like peptide released by Malpighian tubules induces hindgut activity associated to diuresis in the Chagas disease vector Triatoma infestans (Klug) Journal of Experimental Biology 210: 1986-1991. [ Links ]
22. Santini MS, Ronderos JR (2009a). Allatotropin-like peptide in Malpighian tubules: Insect renal tubules as an autonomous endocrine organ. General and Comparative Endocrinology 160: 243-249. [ Links ]
23. Santini MS, Ronderos JR (2009b). Daily variation of an Allatotropinlike peptide in the Chagas disease vector Triatoma infestans (Klug). Biological Rhythm Research 40: 299-306. [ Links ]
24. Scharrer B (1971). Histophysiological studies on the corpora allata of Leucophaea maderae. V. Ultrastructure of cites of origin and release of a distinctive cellular product. Zeitschrift für Zellforschung und mikroskopische Anatomie. 120: 1-16. [ Links ]
25. Scharrer B (1978). Histophysiological studies on the corpora allata of Leucophaea maderae. VI. Ultrastructural characteristics in gonadectomized females. Cell and Tissue Research 194: 533-545. [ Links ]
26. Schooneveld H (1979). Precocene-induced necrosis and haemocyte-mediated breakdown of corpora allata in nymphs of the locust Locusta migratoria. Cell and Tissue Research 203: 25-33. [ Links ]
27. Sedlak BJ (1983). Correlations between endocrine gland ultrastructure and hormone titers in the fifth larval instar of Manduca sexta. General and Comparative Endocrinology 52: 291-310. [ Links ]
28. Steel CGH, Bollenbacher EW, Smith SL, Gilbert LI (1982). Hemolymph ecdysteroid titers during larval adult development in Rhodnius prolixus: Correlations with moulting hormone action and brain neurosecretory cell activity. Journal of Insect Physiology 28: 519-525. [ Links ]
29. Szibbo CM, Tobe SS (1981). Cellular and volumetric changes in relation to the activity cycle in the corpora allata of Diploptera punctata. Journal of Insect Physiology 27: 655-665. [ Links ]
30. Tobe SS, Clarke N, Stay B, Ruegg RP (1984). Changes in cell number of the corpora allata of the coackroach Diploptera punctata: A role for mating and the ovary. Canadian Journal of Zoology 62: 2178-2182. [ Links ]
31. Truesdell PF, Koladich PM, Kataoka H, Kojima K, Suzuki A, McNeil JN, Mizoguchi A, Tobe SS, Bendena WG (2000). Molecular characterization of a cDNA from the true armyworm Pseudoletia unipuncta encoding Manduca sexta allatotropin peptide. Insect Biochemistry and Molecular Biology 30: 691-702. [ Links ]
32. Unnithan GC, Nair KK, Bowers WS (1977). Precocene-induced degeneration of the corpus allatum of adult females of the bug Oncopeltus fasciatus. Journal of Insect Physiology 23: 1081-1094. [ Links ]
33. Vafopoulou X, Steel CGH, Terry KL (2005). Ecdysteroid receptor (EcR) shows marked differences in temporal patterns between tissues during larval-adult development in Rhodnius prolixus: correlations with hemolymph ecdysteroid titers. Journal of Insect Physiology 51: 27-38. [ Links ]
34. Veenstra JA, Costes L (1999). Isolation and characterization of a peptide and its cDNA from the mosquito Aedes aegypti related to Manduca sexta allatotropin. Peptides 20: 1145-1151. [ Links ]
35. Wigglesworth VB (1934). The physiology of ecdysis in Rhodnius prolixus (Hemiptera) II. Factors controlling moulting and metamorphosis. Quarterly Journal of Microscopical Sciences 77: 91-222. [ Links ]
36. Wigglesworth VB (1936). The function of the corpus allatum in the growth and reproduction of Rhodnius prolixus (Hemiptera). Quarterly Journal of Microscopical Sciences 79: 91-121. [ Links ]
37. Wigglesworth VB (1948). The functions of the corpus allatum in Rhodnius prolixus (Hemiptera). Journal of Experimental Biology 25: 1-15. [ Links ]
Received: July 17, 2008.
Revised version received: August 3, 2009.
Acepted: August 12, 2009.














