Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Biocell
Print version ISSN 0327-9545
Biocell vol.34 no.1 Mendoza Jan./Apr. 2010
ORIGINAL ARTICLES
Leaf surfaces of Gomphrena spp. (Amaranthaceae) from Cerrado biome
Suzane Margaret Fank-de-Carvalho, Misléia Rodrigues de Aguiar Gomes, Pedro Ítalo Tanno Silva, Sônia Nair Báo
Departamento de Biologia Celular, Laboratório de Microscopia Eletrônica, Universidade de Brasília, Campus Universitário Darcy Ribeiro, Instituto de Ciências Biológicas. CEP 70919-970, Brasília/DF, Brasil.
*Address correspondence to: Sônia Nair Báo. E-mail: snbao@unb.br
ABSTRACT: The leaf structure and micromorphology characterize plant species and reflex its interactions with the environment. Leaf epidermis sculptures aid high transpiration plants on light reflection. The form and distribution of epicuticular wax crystalloids are important to characterize the surface. Aiming to know the micromorphology and the ultrastructure of G. arborescens, G. pohlii and G. virgata, leaves of these Cerrado native species were collected in Brasília, Distrito Federal, Brazil, at the Olympic Center of the Universidade de Brasília and at Reserva Ecológica do Roncador. Leaves of G. globosa, an Indian native species, were also studied for comparison. Leaves were fractionated, fixed and treated for observation under optical and scanning electron microscope. A description of the leaf epidermis is provided, along with some quantitative data to help the species taxonomy and support future studies on their physiology: all species are amphistomatic and have Stomatal Index between 7.27 and 18.99. The Gomphrena spp. studied have epicuticular wax platelets and wax sculptures over their larger trichome, which are relevant for their taxonomy. Over the Cerrado species cuticle, epicuticular wax is damaged by fungi hyphae development. The presence of epicuticular wax on Gomphrena spp. leaves corroborates the phylogenetical alliance between Amaranthaceae and Chenopodiaceae.
Key words: Anatomy; Epicuticular wax; Platelets; Trichomes; Stomata
Introduction
The Amaranthaceae is a cosmopolite family and occurs at disturbed, arid or saline areas; one of the characteristics that ensure its survival in adverse environments is the operation of C4 pathway of photosynthesis (Judd et al., 1999; Borsch et al., 2001), with the presence of the Kranz syndrome and the characteristic ultrastructure (Carolin et al., 1978; Estelita-Teixeira and Handro, 1984). Other characters could collaborate on the wide distribution of the Amaranthaceae, like lifespan, plants habit and leaf structure, indumentum and venation types (Judd et al., 1999).
A broad definition of the Amaranthaceae family includes Chenopodiaceae, which is supported by morphology, rbcL and ORF2280 sequences, chloroplast structure and its DNA restriction sites, in a total of 2000 species and 170 genera, from which 100 spp. and 20 genera are native of Brazil (Judd et al., 1999; Souza and Lorenzi, 2008). Leaves and roots of some species of the family are edible (Chenopodium spp., Spinacia oleracea, Beta vulgaris and Amaranthus spp.) (Judd et al., 1999). Seeds of South American species of Chenopodium and Amaranthus (quinoa) are used to make flour and others are ornamental, such as Celosia spp., Gomphrena spp. and Iresine spp. (Judd et al., 1999). From a total of 120 spp. of the Gomphrena gender, 46 species occur in Brazil and 41% of it is found in the Cerrado biome (Siqueira, 1992 and 2002; Marchioretto et al., 2002). This biome raises species of the genera Alternanthera, Amaranthus, Chamissoa, Froelichia, Froelichiella, Hebanthe, Pfaffia and Xerosiphon (Siqueira, 1992; Mendonça et al., 1998; Cavalcanti and Ramos, 2001; Marchioretto et al., 2002; Marchioretto, 2008). Twenty species of the family are used in Brazil as food or as popular medicine plant, mostly species from Alternanthera, Amaranthus and Gomphrena genera (Siqueira, 1987). Amaranthaceae species present striated stem and entire alternate or opposite leaves and a great number of them are considered pioneer, growing in clay or sandy soil, frequently among rocks (Joly, 1998; Ribeiro et al., 1999).
The Cerrado biome has a landscape that varies from tall savanna woodland to low open grassland with no woody plants and supports the richest flora among the world's savannas and has a high degree of endemism (Simon and Proença, 2000; Klink and Machado, 2005). This brazilian biome is considered one of the hotspots for the conservation of global biodiversity, with 10.000 plants species (almost 50% of it are endemic), completely adapted to survive adverse conditions of soil and climate, and only 30% of its biodiversity is reasonably known (Myers et al., 2000; Paiva, 2000; Klink and Machado, 2005). Coutinho (1980) believes that frequent fire is one of the most important factors to determine this biome vegetation, alternating communities and maintaining its biodiversity. The expansion of crops at Cerrado biome, which occupies around 40% of its area, has accelerated the natural habitat fragmentation, leading to local biodiversity extinction, introduction of exotic species, soil erosion, water pollution and alterations in vegetation and hydrologic conditions (Miranda and Miranda, 2000; Ratter et al., 2000; Klink and Machado, 2005).
The leaves are the most plastic structures of the plant and their modifications helps to understand how structure, function and metabolism are related, since leaves play a vital role in photosynthesis (Mauseth, 2008). The outermost layer of the leaf is the cuticle, which recovers the epidermis and act as a barrier against mechanical damages, insects, excessive light and loss of water (Bird and Gray, 2003). The cuticle is a film of soluble and polymerized lipids (cutin and suberin) and the wax sculptures that rise from the cuticle plant are influenced by the environment, helping the species to reflect excessive light and to waterproof the epidermis, retaining water and improving the defenses against fungi (Salatino et al., 1986; Rudall, 2007; Mauseth, 2008). Plants can reach the same adaptation pattern by different character combinations. The leaves epidermis is influenced by the plant needs for performing efficient photosynthesis, which includes the compact arrangement of cells and the absence or presence of some epidermal characters, like cuticle and stomata (Esau, 1977; Rudall, 2007; Mauseth, 2008).
Scanning electron microscopy methods are not suitable to detect the thin wax films that are probably present in all terrestrial angiosperms but are good for determining local wax projections and detailed views of the surfaces (Wilkinson, 1979; Engel and Barthlot, 1988). When appears as crystalloids, the individual form, distribution and orientation of the epicuticular wax can be important to characterize the surface as a valuable systematic character (Engel and Barthlott, 1988; Barthlott et al., 1998). The first survey on Amaranthaceae stricto sensu family did not find epicuticular wax on species of the genera Aerva, Allmania, Alternanthera, Amaranthus, Celosia, Deeringia, Gomphrena, Iresine and Ptilotus, while Chenopodiaceae members presented small epicuticular platelets (minor than 2 mm) with parallel orientation and not restricted to stomata neighbourhood (Engel and Barthlot, 1988).
In order to improve the knowledge of the biodiversity of the Cerrado biome and to understand the structural characteristics plants developed on evolutionary adaptations to this environment, the leaf epidermis of three Cerrado native species (Gomphrena arborescens L.f., G. pohlli Moq. and G. virgata Mart.) and of one cultivated plant - G. globosa L. - where structurally described under optical and scanning electron microscopy in search for taxonomically and environmentally relevant characteristics.
Material and Methods
Data research on native species and sample collection
Along with a survey on Herbaria data (Herbarium of the Universidade de Brasília/UB, Herbarium of Reserva Ecológica do Roncador/IBGE, Herbarium of the Universidade Vale dos Sinos/PACA and Herbarium of Empresa Brasileira de Pesquisa Agropecuária/CEN), numerous field searches were carried out in cerrado sensu stricto areas of Distrito Federal (Parque Ecológico Olhos D´água, Reserva Ecológica do Roncador, Centro Olímpico of Universidade de Brasília and other areas on road vicinities of Park Way and Jardim Botânico) in order to locate the native species in the Cerrado biome.
The Cerrado biome is a tropical savanna-like ecosystem that occupies about 2 millions of km" (from 3-24º S and from 41-43º W), presenting a hot, semi-humid, and notably seasonal climate, with a dry winter season and a rainy summer, raising typical vegetation (Dias, 1992; Simon and Proença, 2000; Eiten, 2001).
Leaves of the 3rd to 5th nodes of the Cerrado native adult species were collected in the cerrado sensu stricto areas of the Olympic Center of the Universidade de Brasília (G. arborescens, G. pohlii and G. virgata) near the coordinates 15º45.936' S and 047º51.187' W and at the Reserva Ecológica do Roncador - RECOR/IBGE (G. arborescens and G. virgata), both in Brasília/DF, while the similar material of G. globosa was harvest from public gardens, in Brasília/DF, near 15º45.950' S and 047º53.006' W coordinates. Testimony material was deposited at the IBGE, PACA and UB Herbaria. Leaves of the 3rd to 5th nodes of G. arborescens juvenile species (6-9 months old), cultivated from seeds at vegetation house conditions were also collected and submitted to the same treatment as the adult ones.
Sample and methodology
The species leaves were fractionated and the middle part of them was fixed in Karnovsky solution (Karnovsky, 1965) and in FAA70 or FPA solutions (Kraus and Arduin, 1997) for 24 h. The Karnovsky fixed sample was post fixed in 1% osmium tetroxide in 0.05M sodium cacodylate buffer pH 7.2 for 1 h. Small pieces of fresh tissue were directly fixed over a stub for scanning electron microscopy and air dried without any preparation, as control pieces (Paiva, 2005). Part of the material of both fixatives were dehydrated in a series of ascending acetone and/or ethanol gradient (30%-100%) and critical point dried to the in a Critical Point Drier CPD 030 (Balzers) and, along with air dried samples, were gold sputtered in a SCD 050 (Balzers), followed by the observation under the scanning electron microscope JEOL JSM 840-A.
Part of the material of FAA70 fixative was hand free sliced and submitted to a Sudan III histochemical test to detect lipids, cutin and suberin on the epidermis of the leaves and to a solution of acid floroglucin to detect lignin (Kraus and Arduin, 1997). Other leaf rectangular pieces were washed in distilled water and submitted to 50% nitric acid solution and boiled until the mesophyll was dissolved, sparing the adaxial and abaxial epidermis apart. Then, the epidermis was washed again, stained with safranin and mounted between histological slides and cover slips with Kayser´s glycerinated gelatin (Kraus and Arduin, 1997). As this technique was not good enough for all the studied species, some pieces of the leaves were cleared with a sequence of sodium hydroxide and sodium hypochlorite solutions, followed by safranin staining (Kraus and Arduin, 1997) and mounted between glass pieces with Verniz Vitral Incolor of Acrilex™ (Paiva et al., 2006). All samples were observed under a Zeiss Axiophot equipped with AxioCam and the AxioVision software.
Stomata and trichome density were determined by counting random areas of cleared leaves and expressed as the médium number by mm2 plus the standard deviation on adaxial and abaxial epidermis surfaces of at least three leaves. The Stomatal Index were determined for all the four species on the middle adaxial and abaxial epidermis of the same leaf surfaces, calculating the percentage of stomata in relation of the other epidermal cells as SI = [S/(E+S)]*100, where SI is the Stomatal Index, S is the number of Stomata per unit area and E is the number of Epidermal cells (including basal cells of trichomes) per same unit area (Metcalfe and Chalk, 1979).
The areas occupied by the ordinary epidermal cells, by the basal cell of the largest trichome and by the stomata complex (formed only by the 2 guard cells) were measured with the AxioVision software, on the adaxial epidermis surface at the middle part of the leaves, in 25 cells of each type. The results were expressed as the μm2 medium area plus standard deviation. The larger trichome length of each species was measured at 25 of these epidermis appendixes, and length was expressed in µm plus standard deviation (Theobald et al., 1979).
Results
Species origin, morphology and phenology
Three of the Gomphrena species used in this study are Brazilian Cerrado native species: Gomphrena arborescens L.f., G. pohlii Moq. and G. virgata Mart. The fourth species, Gomphrena globosa L., is an annual species, native from India and cultivated as ornamental. All the Cerrado Gomphrena species can be classified as hemicryptophytes as they are perennial, the renewal buds are located on top of storage roots, the aerial parts dry out in the dry season (Fahn and Cutler, 1992) and the morphology of the subterranean organs suggest they have xylopodia. Leaves are opposite and hairy, with mucronate apex and entire margins. Cerrado species have sessile leaves, while G. globosa leaves are petiolate. During the dry season the seeds of the Cerrado species are dispersed, what seems appropriate since all of them are dispersed by the wind.
Gomphrena arborescens L.f. (Fig. 1A) is known as paratudo-do-campo and is a subshrub of 50 cm high, with coriaceous leaves of 10 cm long and 7 cm wide, found in the mid-west and southeast regions of Brazil and in Paraguay and used in folk medicine and in dried flowers arrangements (Barros, 1982; Pio Corrêa, 1984; Siqueira, 1987, 1992, 2002; Salles and Lima, 1990; Lorenzi and Matos, 2002). The species was ound at bloom time at RECOR/IBGE and at the Olympic Center of Universidade de Brasília, in Brasília/DF, usually in the rainy station (from November to April) and at vegetative stage from August to October. Some variations of the leaf position were found on G. arborescens, from alternated just below the apical inflorescence to opposite towards the base in adult Cerrado native plants, tending to be decussate. The leaves of a young plant growing at a vegetation house were always decussate. In this species, leaves are always attached to the stem at the same time as the flowers and fruits. Usually, during mostly of the dry season its aerial parts dry out.

FIGURE 1. Studied species. Figures 1A to 1E: Brazilian Cerrado native species. Figure 1F: Indian native species,
cultivated in Brazil. 1A: Gomphrena arborescens L.f., known as paratudo-do-campo, is a subshrub of 50 cm high,
with coriaceous leaves. 1B: Inflorescence of G. pohlii Moq., known as paratudinho, a subshrub of 180 cm high. 1C: G. pohlii Moq. coriaceous leaves. 1D: Inflorescence of G. virgata Mart., known as cangussu-branco, a subshrub of
200 cm high. 1E: G. virgata linear coriaceous leaves. 1F: G. globosa L., known as perpétua, is a ornamental herb
with membranaceous leaves. Bar = 1 cm.
G. pohlii Moq. (Fig. 1B - flowers and Fig. 1C - leaves) is a subshrub of 180 cm high with coriaceous leaves of 10 cm long and 7 cm wide, found in the mid-west and southeast regions of Brazil and in Paraguay and known as paratudinho, also used in folk medicine (Siqueira, 1988, 1992). The species was found only at the Olympic Center of Universidade de Brasília, in Brasília/DF, at the same time as G. arborescens. The phenologies of both species are related and leaves are always attached to the
stem, at the same time as the flowers and fruits. The bloom time occurs from December to April and the species was found at vegetative stage from August to November. During the dry season the aerial parts of the plant dry out.
G. virgata Mart. (Fig. 1D - flowers and Fig. 1E - leaves) is a subshrub of 200 cm high with linear coriaceous leaves of 15 cm long and 1 cm wide, broadly distributed in Brazil and known as cangussu-branco, also used in folk medicine (Pio Corrêa, 1984; Siqueira, 1992). The species was found at RECOR/IBGE and at the Olympic Center of Universidade de Brasília, in Brasília/DF, from March (end of the rainy season) to July in vegetative stage. The bloom time of the species begins at July (middle of the dry season) and fructification goes until September (end of the dry season). During the bloom period, leaves enter in a senescence process and are disposed by the stem. After the blooming and dispersing the seeds, the aerial parts of the species also dry out, until almost the end of the rainy season (around March), when they re-sprout.
G. globosa L. (Fig. 1F) is a small herb with membranaceous leaves about 10 cm long and 4 cm wide, native of India and known in Brazil as perpétua, cultivated as ornamental (Siqueira, 1992; Lorenzi and Souza, 1995), including in Brasília squares and gardens, at the core region of the Cerrado biome. The species was collected in January, during the rainy season, when it is cultivated in public gardens of Brasília.
Anatomy
The studied species have uniseriate epidermis and tomentose amphistomatic leaves, with anomocytic stomata dispersed over the whole adaxial surface, except over the midrib and on the leaf ledges, where cells are elongated. Trichomes are always nonglandular and have at least two sizes and shapes. On the abaxial surface, stomata do not occur over the midrib and secondary veins, where cells are elongated, as well as over elongated cells that reinforce the leaves edges. All the studied species present stomata surrounded by three to five cells, but these cells are not different from other ordinary epidermal cells, so they were not considered as stoma subsidiary cells.
On paradermal view, G. globosa (Fig. 2A) presents the highest stomatal index of all the studied species (Table 1), followed by G. arborescens (Fig. 2B), G. pohlii (Fig. 2C) and G. virgata (Fig. 2D). G. arborescens and G. pohlii presents the highest number of cell per area unit (Table 1), but G. arborescens presents the largest area occupied by just one epidermal cell (Table 2). G. virgata presents the minor number of cells by area (Table 1), and the major medium area occupied by each ordinary epidermal cell and stomata complexes (Table 2).
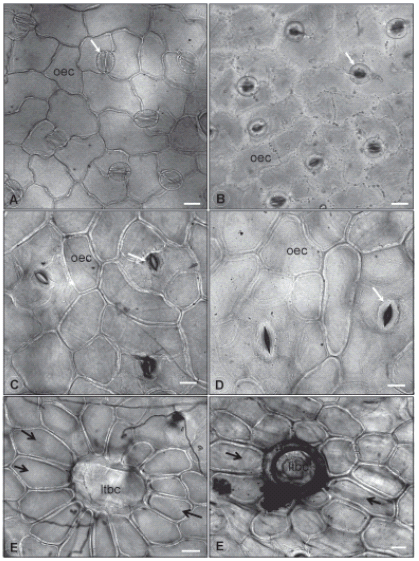
FIGURE 2. Leaf epidermal
surfaces under optical
microscope. 2A: Abaxial
surface of G. globosa leaf.
2B: Abaxial surface of
G. arborescens leaf. 2C: Abaxial surface of G. pohlii leaf. 2D: Abaxial surface of
G. virgata leaf. 2E: Long trichome
basal cell and subsidiary
ones on G. pohlii leaves. 2F: Long trichome
basal cell and subsidiary
ones on G. virgata leaves.
Legend: ltbc: long trichome
basal cell; oec: ordinary epidermal
cell; black arrow: trichome
subsidiary cell; white
arrow: stoma. Bar = 20 μm.
TABLE 1. Quantitative data on trichome and stomata density and Stomatal Index
(SI) of both surfaces of the studied leaves.

TABLE 2. Quantitative data on area occupied by each kind of epidermis cell and
size of stoma and large trichome of the studied leaves.

The ordinary epidermal cells are polygonal in all four species, but their anticlinal walls are thicker and have a straight path on both epidermis surfaces of G. virgata (Fig. 2D, F) and G. pohlii (Fig. 2C, E) leaves. G. virgata also presents elongated epidermal cells around the basal cells of its trichomes. The epidermis leaves of G. arborescens have more sinuous anticlinal walls on ordinary epidermal cells of abaxial surface (Fig. 2B), while the same cells on adaxial surface have straight anticlinal walls. Thicker anticlinal walls are restricted to the elongated cells that recover the edges and the major veins on G. globosa epidermis and this species also tends to have more sinuous anticlinal walls on ordinary epidermal cells towards the edges of both surfaces (Fig. 2A) and more straight anticlinal walls towards the mid vein.
Around the longer nonglandular trichomes of G. arborescens, G pohlii (Fig. 2E) and G. virgata (Fig. 2F), the epidermal cells have rectangular gross shape, thicker anticlinal cell walls and are lignified, what could characterize a kind of trichome subsidiary cells, specially in G. arborescens and G. pohlii, which are very similar in shape and disposition. This aspect was not found on G. globosa leaves.
Analyzing leaf transversal sections (data not showed), the Cerrado native species G. arborescens, G. pohlii and G. virgata have a thicker cuticle than the cultivated species G. globosa and than a juvenile sample (6-9 months old) of G. arborescens cultivated under vegetation house conditions. The two stoma guard cells have prominent outer and small inner ledges. Cutin and suberin are restricted to the periclinal external wall of most of the epidermal cells, except at G. virgata epidermis, where the water proof cuticle extends itself towards to the inner sub-stomatal chamber, covering also the anticlinal wall of the guard cell and the proximal cells on both epidermis surfaces (adaxial and abaxial).
Micromorphology
All the studied species present a thin film of wax covering the cuticle of the outermost wall of the epidermal cells and appendixes, sometimes superimposed by crystalloids of epicuticular wax. The cuticle has striations over the first and second order veins and on the edge of the leaves (abaxial surface) and over the mid vein (adaxial surface) of all species, markedly on Cerrado species (Fig. 3A).

FIGURE 3. Leaf epidermis
and stomata under scanning
electron microscopy.
3A: G. virgata adaxial epidermis
with striae also over
the secondary order vein
(white arrow) and fungi
hyphae (arrow head). 3B: G. arborescens stoma and
epicuticular wax (black arrow).
3C: G. pohlii stoma.
3D: G. virgata stoma. 3E: G. globosa stoma. Legend: ltbc: long trichome
basal cell. Bars: 3A = 100μm; 3B to 3E = 10 μm.
In the studied species the stomata guard cells are leveled to the surrounding cells (Fig. 3A-D), tending to be more elevated on abaxial surface. The anomocytic stomata of the four species have guard-cells with prominent outer ledges (Fig. 3B-D). Only on G. virgata adaxial surface the stomata are slightly depressed between the ordinary epidermal cells. The guard cells of G. arborescens have ledges with thin and irregular borders (Fig. 3B) and there are some particles of epicuticular wax nearby, some recognizable as platelets. The ledge borders of G. pohlii guard cells are thin and regular and near its stomata some perpendicular ridges and crystals of epicuticular wax can be found (Fig. 3C). The stoma of G. virgata has ledges with regular and thick borders and processing artifacts do not allow epicuticular waxes to be observed (Fig. 3D). The ledges of the stoma guard cells of G. globosa have thick and regular borders and it is possible to see that the epidermal outer cell walls of neighbors' cells are convex (Fig. 3E). This last feature was not observed for any other of the studied species, which epidermal outer cell walls tend to be flat.
Over the elongated epidermal cells, that reinforces the leaf edges and veins, both types of trichomes are more frequent and there are no stomata (Fig. 4A). There are two types of nonglandular trichomes: a long uniseriate hair with osteolate thick cell walls and acuminate apex (Fig. 4A, Table 2) and a short uniseriate recurved hair with thin cell walls and a soft (not acuminate) apex (Fig. 4B).

FIGURE 4. Leaf trichomes under scanning electron microscopy. 4A: G. globosa abaxial surface showing a large trichome
(arrow head) and elongated cells over the veins (star). Sample fixed in Karnovsky mix and dehydrated in
ethanol. 4B: Short trichome of G. arborescens with no wax ornamentation and non nodose articulation (ellipsis). Sample
fixed in FAA70 and dehydrated in ethanol. 4C: G. arborescens long trichome epicuticular wax ornamentation near a
nodose articulation (ellipsis) and the smallest teichodes (arrow). Sample not fixed. 4D: G. pohlii long trichome epicuticular
wax scarce striation, the largest teichodes (arrow) and a nodose articulation (ellipsis). Sample not fixed. 4E: G.
virgata long trichome densely ornamented by epicuticular wax striations, few intermediary sized teichodes (arrow) and
a nodose articulation (ellipsis). Sample not fixed. 4F: G. globosa long trichome presents only few intermediary sized
teichodes (arrow) and nodose articulation (ellipsis). Sample not fixed. Bars: 4A = 100 μm; 4B to 4F = 10 μm.
The longest trichomes of the four species have similar morphology: round basal cells, pustulate osteolate intermediate cells with nodose articulation and acuminate apical cell (Fig. 4A). This trichome type usually has live basal cells (all the 2-3 round ones and at least one of the elongated intermediate cells) and these cells are translucent. The intermediate and apical cells give the trichome color: white in G. globosa and rusty (brown-orange) in the three Cerrado species.
The short trichomes present smaller and usually collapsed cell walls and there is no epicuticular wax ornamentation (Fig. 4B) over it or on either of the other species. This kind of trichome is formed only by living and thin walls translucent cells and is usually destroyed by fungi hyphae when the plant is living at the Cerrado biome. The articulation of the uniseriate cells is always simple, not nodose (Fig. 4B).
The large trichome presents no epicuticular wax ornamentation over the basal and round cells. However, over the anticlinal walls of the osteolate intermediate and apical cells a network of epicuticular wax streaks and teichodes can be present. This can be useful to discriminate species, because the morphology and ornamentation is slightly different in each one of them. The osteolate cells of the larger trichome of G. arborescens have dense epicuticular wax striations with small and rare teichodes (Fig. 4C). These cells in G. pohlii trichome have a few epicuticular wax striations and well developed but scarce teichodes of around 10 mm long (Fig. 4D). The same cells in G. virgata trichome have dense epicuticular wax striations and rare small teichodes (Fig. 4E). G. globosa cells have no epicuticular wax striations and have the greatest amount of teichodes of intermediate size (Fig. 4F). In all the four species, the articulation between the intermediate osteolate cells is nodose, characterized by the interlaced digitorum projections of the periclinal cell walls of neighboring cells (Fig. 4C-F).
G. arborescens leaves present the greatest amount of epicuticular wax particles over the ordinary epidermal cells, normally as entire parallel platelets (Fig. 5A), all over the leaves. Typically, over the subsidiary cells of the largest trichome and sometimes near or over stomata, the platelets can be disposed as single arrows radiating to the adjacent cells (Fig. 5A). This epicuticular wax disposition was not found in any of the other species of the study. Non entire platelets, granules and rosettes were also found (Fig. 5B). Epicuticular wax over guard cells of G. arborescens stoma is less concentrated and can be disposed in different arrangements. Platelets also can have irregular margins. G. arborescens epicuticular waxes had been damaged by fungi hyphae (Fig. 5A).

FIGURE 5. Epicuticular waxes over leaf cuticles under scanning electron microscopy. 5A: G. arborescens abaxial surface
with fungi hyphae (h) and epicuticular wax organized as a row of parallel platelets (arrow) near a stoma (s) and damaged
by a detached hyphae (arrow head) on sample fixed with FPA and dehydrated with ethanol. 5B: G. arborescens adaxial
surface epicuticular wax granules (arrow) and rosettes (ellipsis) on control sample (not fixated). 5C: G. pohlii epicuticular
waxes on adaxial surface a control sample (not fixed). 5D: G. virgata epicuticular waxes near the stoma (s) on adaxial
surface of a control sample (not fixed). Bar = 10 μm.
G. pohlii leaves present rare platelets of epicuticular wax (Fig. 5C), more frequently over the ordinary epidermal cells near the stomata and over the guard-cells. On G. virgata ordinary epidermal cells, the rare epicuticular wax crystals were restricted to the stomata neighbor cells of the adaxial surface of the leaf epidermis (Fig. 5D). Epicuticular wax particles are very rare over G. globosa leaf cuticles (data not shown). The three species of the Brazilian Cerrado presented high fungal contamination (Fig. 4A and 5A) while G. globosa did not.
Discussion
Although all the Gomphrena Cerrado species have coriaceous leaves and share the same seasonally dry habitat, the anatomical data suggest no water restriction in these organs. All the Gomphrena Cerrado species vegetate under full sunlight, in open environment and tend to have from medium to large stomata, leveled to the other epidermal cells. According to Wilkinson (1979) larger stomata are coincidental with full sunlight and drier conditions while smaller stomata are coincidental with shade, humid atmosphere and moist soil conditions.
The Cerrado Gomphrena spp. present xeromorphic features as thick cuticle and a kind of hypodermis under the larger trichomes, as verified in G. arborescens L.f. leaves (Fank-de-Carvalho and Graciano-Ribeiro, 2005). Only in G. virgata the stomata are slightly sunken while on the other species it is leveled to the other epidermal cells on adaxial surface. G. virgata has narrower leaves and aerial parts that vegetate longer during the Cerrado dry season. Sunken stomata are considered important to minimize water loss while the thick cuticle and hypodermis diminish the intensity of light to the photosynthetic tissue (Rudall, 2007), although in G. arborescens it was suggested this tissue could help the light diffusion under the large trichome basal cells and to reinforce the epidermis to maintain this appendix (Fank-de-Carvalho and Graciano-Ribeiro, 2005).
The Amaranthaceae species of this study present similar Stomatal Index (SI) and number of cells per unit of area of the ones studied by Padmini and Rao (1995). Although they found SI of 19,5 on abaxial surface and 18,2 on adaxial surface and our data corresponds to 18,7 and 18,99 for the same surfaces (Table 1). This variation could be explained by the differences of the surrounding environment that plants cultivated in Brazil and in India are subjected to.
The difference between the G. arborescens and G. pohlii are smaller than the difference between both species and G. virgata on the SI and number of cells per area. When these data are related to the size and shape as well as the duration of the leaves of the Cerrado species, they could suggest that the briefer is the lifetime of the organ
and smaller the width of the leaf, smaller the number of epidermal cells per square millimeter and the stomatal index (Table 1), leading to increase the area occupied by each cell. So, if larger cell area implies in bigger water loss (Fahn and Cutler, 1992), it is important to add to this relation the morphology of the roots of these species: all of them have well developed subterranean systems (Siqueira, 1992) which guarantee water supply during the aerial re-sprout and flowering for G. arborescens and G. pohlii and re-sprout of G. virgata (since the latter species looses leaves during the bloom dry period). Even for G. globosa, that has a shorter lifetime and is not perennial, as the other species, this statement can be verified: it has the highest Stomatal Index, the second smaller leaf width, and the second medium number of epidermal cells per area.
All the Gomphrena species studied presented anomocytic stoma. There is a literature controversy about which stomata type is primitive and which is derivate (Wilkinson, 1979) so this classification was used only for diagnostic purposes. The stoma size is medium for most of the species (G. arborescens, G. globosa and G. pohlii), since the length of them is among 26-31 micrometers. Only G. virgata stoma can be considered large, with 38 micrometers of medium length. In this study, the three species with medium sized stoma have similar densities of them by square millimeter, while the species with larger stoma has the smaller density. Our data agrees with Wilkinson (1979), who verified that most authors agree that stoma size is stable enough to be used as a diagnostic character and that small stomata are correlated with high density while large stomata are correlated with low density.
All the species of this study have trichome over the foliar surfaces. The density and length of them is variable (Tables 1 and 2) and is interesting to note that the translucent and larger cells are near the leaf surface. G. arborescens leaves have the larger basal cells that bear the lengthiest trichomes and one of the smaller densities of this appendages among the studied species. The Brazilian Gomphrena spp. species will be studied in the future to verify if there are other anatomical shared features, as for the trichome types. According to Theobald et al. (1979), the trichome type is often valuable for taxonomic purposes, and the provided electron scanning microscopy figures and descriptions can be useful to identify the species.
The foliar surface studies under the scanning electron microscope revealed the existence of crystalloid wax projections on both foliar surfaces of G. arborescens, with greater concentration on the adaxial one. All the four species presented platelets of epicuticular wax over the periclinal outer wall of the leaf epidermal cells, although in different patterns and density. The streaked cuticle over the first degree venation and borders of Cerrado Gomphrena spp. leaves must represent a way to guarantee the integrity of its epidermis on great tension zones, the same way as the elongated shape of some ordinary epidermal cells and the thicker anticlinal walls of the same cells.
When studying Cerrado species, sometimes it can be necessary to cultivate them in a controlled environment to get a better description of the epicuticular wax patterning, since fungi hyphae can difficult this objective. The Cerrado environment, moist and hot during the rainy season, favors the fungi development over leaves. The micromorphology of juvenile and adult G. arborescens leaves was very similar. As the individual form of the epicuticular wax crystalloids, their distribution and orientation can characterize the surface of the species (Engel and Barthlott, 1988; Barthlott et al., 1998), the juvenile leaves were chosen to study the distribution pattern of epicuticular waxes. Engel and Barthlott (1988) studied Amaranthaceae, including some Gomphrena spp. and did not find epicuticular waxes. They considered the absence of these structures a feature to distinguish Amaranthaceae and Chenopodiaceae, as the later one always presented small and lobed platelets of epicuticular wax, normally with parallel orientation and not restricted to the stomata vicinity.The methodology of sample preparation was very important in relation to epicuticular wax findings. Most of the studied species showed no epicuticular wax
when dehydrated with ethanol or acetone, even if fixated with glutaraldehyde or formaldehyde plus ethanol. Only G. arborescens epicuticular waxes were more resistant to the dehydration process, using both acetone and ethanol, but the best preservation of it was also observed in the control sample. So, the use of the control sample, just dried out over a stub, was very relevant for this study. The use of control sample must be complimentary to the use of fixated sample, because there is no preservation of the shape of the ordinary epidermal cells and stomata. There was found no significant difference only due to the fixative used on our sample.
The presence of epicuticular wax did not prevent the development of fungi hyphae on leaves of none of the three Cerrado
native species. There were hyphae all
over the epidermis, sometimes penetrating into the mesophyll
through some epidermal cells, walls of the smallest
trichomes and stomata pores. Usually the epidermis
is damaged near the fungi infected areas and, under the
hyphae, the epicuticular wax can loose its shape or melt
(Fig. 5A). The leaf surface sculptures should improve
the protection of the surface against fungi infections
(Salatino et al., 1986), and maybe this is one of the strategies
of the Cerrado species, which present more sculptures
and epicuticular waxes in relation to G. globosa.
Cerrado Gomphrena spp. must have adapted to the
Cerrado environment through the strategy of enhancement
of the photosynthesis process since they do not
present a significative reduction on the leaf area or on
the epidermal cells sizes. Besides, the indumentum of
these species is not so dense. They present amphistomatic
leaves and Stomatal Index or stomatal density similar
or higher than other species of higher photosynthetic
rates, like the C4 G. globosa (Padmini and Rao, 1995)
and Zea mays (Mauseth, 2008). Photosynthesizing organs
can present two strategies of adaptation to arid
conditions and the second strategy would be the reduction
in the transpiration rate (Fahn and Cutler, 1992),
what implicates in the study of the mesophyll structure,
which is on development. On the other hand, these species
have well developed cuticle and thick anticlinal
walls and the structure of the epicuticular wax tends to
be of thin plates, features considered xeromorphic and
inducible by drought (Fahn and Cutler, 1992).
Conclusions
Two of the Cerrado species can be misidentified
during the very early vegetative stage (G. arborescens and G. pohlii), due to the similarity of their stem and
leaves. This study shows differences between the epidermal
cells and other anatomic and micromorphology
features of the epidermis that can be useful to better
identify the species. The quantitative data for all four
studied species complete the description and can represent
good indicatives for the physiology of the species.
The finding of epicuticular wax in all the
Gomphrena spp. leaves studied reinforces the
phylogenetical relationship between Chenopodiaceae
and Amaranthaceae families, which has been treated
as Amaranthaceae sensu lato. The platelets over the
cuticle did not represent an effective barrier against
fungi hyphae penetration, although it must have improved
it. These results indicate the need of increasing
studies on other species of the Amaranthaceae sensu
lato family, especially the Cerrado native ones, to improve
the understanding of the taxonomy value of the
sculptures arising from the leaf surfaces and its relationship
with the plant adjustment to the environment.
This study shows that the Brazilian Cerrado biome
raises unique vegetation with different strategies to
adapt themselves to the environment, not only structural
but also phenological. All the studied Cerrado
species vegetate under full sunlight (open environments
of the Cerrado biome) and present leaves only during
the rainy season, even though stem and flowers can be
found still green during the dry season. So, all the
leaves have a good supply of water, even though the
atmosphere is not always humid during these days,
because the rainy season coincides with the warmer
tropical and sunny days. Plant life strategy must be
correlated with the morphology and the structural data,
as Stomatal Index, so these data can be correlated with
the species physiology.
Aknowledgments
The authors wish to thank the staff of the Electronic Microscopy Laboratory of the Universidade de Brasília, where the work was carried out, and to CNPq, CAPES, FINEP, RECOR/IBGE and Herbaria IBGE, PACA, UB and CEN. S. M. Fank-de-Carvalho is a student of the Programa de Doutorado em Biologia Celular e Estrutural, Universidade Estadual de Campinas - UNICAMP.
References
1. Barros MGAE (1982). Plantas medicinais - usos e tradições em Brasília - DF. Oréades 8: 140-149. [ Links ]
2. Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998). Classification and terminology of plant epicuticular waxes. Botanical Journal of the Linnean Society 126: 237-260. [ Links ]
3. Bird SM, Gray JE (2003). Signals from the cuticle affect epidermal cell differentiation. New Phytologist 157: 9-23. [ Links ]
4. Borsch T, Clemants S, Mosyakin S (2001). Symposium: Biology of the Amaranthaceae-Chenopodiaceae alliance. Journal of the Torrey Botanical Society 128: 234-235. [ Links ]
5. Carolin RC, Jacobs SWL, Vesk M (1978). Kranz cells and mesophyll in the Chenopodiales. Australian Journal of Botany 26: 683-698. [ Links ]
6. Cavalcanti TB, Ramos AE (orgs.) (2001). Flora do Distrito Federal, Brasil. Embrapa Recursos Genéticos e Biotecnologia, Brasília. [ Links ]
7. Coutinho LM (1980). As queimadas e seu papel ecológico. Brasil Florestal 10: 7-23. [ Links ]
8. Dias BFS (coord.) (1992). Alternativas de desenvolvimento dos cerrados: manejo e conservação dos recursos naturais renováveis. FUNATURA/IBAMA, Brasília. [ Links ]
9. Eiten G (2001). Vegetação natural do Distrito Federal. SEBRAE/UnB, Brasília. [ Links ]
10. Engel T, Barthlott W (1988). Micromorphology of epicuticular waxes in Centrosperms. Plant Systematics and Evolution 161: 71-85. [ Links ]
11. Estelita-Teixeira ME, Handro W (1984). Leaf ultrastructure in species of Gomphrena and Pfaffia (Amaranthaceae). Canadian Journal of Botany 62: 812-817. [ Links ]
12. Esau K (1977). Anatomy of Seed Plants, second ed. John Wiley and Sons, New York. [ Links ]
13. Fanh A, Cutler DF (1992) Xerophytes. Gebrüder Borntraeger, Berlin. [ Links ]
14. Fank-de-Carvalho SM, Graciano-Ribeiro D (2005). Arquitetura, anatomia e histoquímica das folhas de Gomphrena arborescens L.f. (Amaranthaceae). Acta Botanica Brasilica 19: 379-392. [ Links ]
15. IBGE (2004). Reserva Ecológica do IBGE - ambiente e plantas vasculares. Vol. 3 - Estudos e Pesquisas - Informação Geográfica. MPOG/IBGE, Rio de Janeiro. [ Links ]
16. Joly AB (1998). Botânica: introdução à taxonomia vegetal. 12 ed. Companhia Editora Nacional, São Paulo. [ Links ]
17. Judd WS, Campbell CS, Kellog EA, Stevens PF (1999). Plant Systematics - a phylogenetic approach. Sinauer Associates, Sunderland. [ Links ]
18. Karnovsky MJ (1965). A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy, Abstracts Fifth Annual Meeting American Society for Cell Biology. Journal of Cell Biology 27: 137-138A. [ Links ]
19. Klink CA, Machado RB (2005). A conservação do Cerrado brasileiro. Megadiversidade 1: 147-155. [ Links ]
20. Kraus JE, Arduin M (1997). Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro. [ Links ]
21. Lorenzi H, Matos FJA (2002). Plantas medicinais no Brasil-nativas e exóticas. Instituto Plantarum, Nova Odessa. [ Links ]
22. Lorenzi H, Souza HM (1995). Plantas ornamentais no Brasil-arbustivas, herbáceas e trepadeiras. Instituto Plantarum, Nova Odessa. [ Links ]
23. Marchioretto MS, Windisch PG, Siqueira JC (2002). Os gêneros Froelichia Moench e Froelichiella R.E. Fries (Amaranthaceae) no Brasil. Pesquisas, Botânica 52: 7-46. [ Links ]
24. Marchioretto MS (2008). Os gêneros Hebanthe Mart. e Pfaffia Mart. (Amaranthaceae) no Brasil. Thesis, Universidade Federal do Rio Grande do Sul. [ Links ]
25. Mauseth JD (2008). Botany - an introduction to plant biology, 4th ed. Jones and Bartlett Pub., Sudbury. [ Links ]
26. Mendonça RC, Felfili JM, Walter BMT, Silva MC, Rezende AR, Filgueiras TS, Nogueira PE (1998). Flora vascular do Cerrado, in Sano SM, Almeida SP (Eds.) Cerrado: ambiente e flora. Embrapa CPAC, Planaltina, pp 286-556. [ Links ]
27. Metcalfe CR, Chalk L (1979). Anatomy of the Dicotyledons- vol. I - Systematic Anatomy of the leaf and stem, second edition. Oxford University Press, Suffolk. [ Links ]
28. Miranda H, Miranda AC (2000). Queimadas e estoques de carbono no Cerrado, in Moreira AG, Schwartzman S (eds) As Mudanças Climáticas e os Ecossistemas Brasileiros. Foco, Brasilia, pp 75-81. [ Links ]
29. Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000). Biodiversity hotspots for conservation priorities. Nature 403: 853-858. [ Links ]
30. Padmini S, Rao RS (1995). Structure, distribution and taxonomic importance of foliar stomata in some Indian Amaranthaceae. Botanical Journal of the Linnean Society 118: 149-161. [ Links ]
31. Paiva EAS (2005). Effect of sample preparations for SEM studies of epicuticular wax in Tradescantia pallida (Commelinaceae) leaves. Brazilian Journal of Morphological Sciences supplement 2005: 258. [ Links ]
32. Paiva PHV (2000). A Reserva da Biosfera do Cerrado: fase II, in Cavalcanti TB, Walter BMT (eds) Tópicos atuais em botânica - palestras convidadas do 51° Congresso Nacional de Botânica. Sociedade Brasileira de Botânica/Embrapa-Cenargen, Brasília, pp 332-334. [ Links ]
33. Paiva JGA, Fank-de-Carvalho SM, Magalhães MP, Graciano-Ribeiro D (2006). Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Botanica Brasílica 20: 257-264. [ Links ]
34. Pio Corrêa M (1984). Dicionário de plantas úteis do Brasil e das exóticas cultivadas. IBDF, Rio de Janeiro. [ Links ]
35. Ratter JA, Ribeiro JF, Bridgewater S (2000). Woody flora distribution of the Cerrado Biome: phytogeography and conservation priorities, in Cavalcanti TB, Walter BMT (eds) Tópicos atuais em botânica - palestras convidadas do 51° Congresso Nacional de Botânica. Sociedade Brasileira de Botânica/Embrapa-Cenargen, Brasília, pp 340-342. [ Links ]
36. Ribeiro JELS, Hopkins MJG, Vicentini A, Sothers CA, Costa MAS, Brito JM; Souza MAD, Martins LHP, Lohmann LG, Assunção PACL, Pereira EC, Silva CF, Mesquita MR, Procópio LC (1999). Flora da Reserva Ducke. Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia Central. INPA, Manaus. [ Links ]
37. Rudall P (2007). Anatomy of flowering plants - an introduction to structure and development. Cambridge University Press, New York. [ Links ]
38. Salatino A, Montenegro G, Salatino MLF (1986). Microscopia eletrônica de varredura de superfícies foliares de espécies lenhosas do cerrado. Revisa Brasileira de Botânica 9: 117-124. [ Links ]
39. Salles AEH, Lima CG (1990). Flores dos Cerrados - Pequeno Guia. GDF, Brasília. [ Links ]
40. Simon MF, Proença C (2000). Phytogeographic patterns of Mimosa (Mimosoideae, Leguminosae) in the Cerrado biome of Brazil: an indicator genus of high-altitude centers of endemism? Biological Conservation 96: 279-296. [ Links ]
41. Siqueira JC (1987). Importância alimentícia e medicinal das amarantáceas do Brasil. Acta Biologica Leopoldensia 9: 99-110. [ Links ]
42. Siqueira JC (1988). Plantas medicinais - identificação e uso das espécies dos cerrados. Edições Loyola, São Paulo. [ Links ]
43. Siqueira JC (1992). O gênero Gomphrena L. (Amaranthaceae) no Brasil. Pesquisas, Botânica 43 : 5-197. [ Links ]
44. Siqueira JC (2002). Amaranthaceae, in Wanderley MGL, Shepherd GJ, Giulietti AN, Melhem TSA, Dittrich V, Kameyama C. Flora fanerogâmica do Estado de São Paulo, vol. II., HUCITEC, São Paulo, pp 11-30. [ Links ]
45. Souza VC, Lorenzi H (2008). Botânica Sistemática - Guia ilustrado para identificação das famílias de Fanerógamas nativas e exóticas do Brasil, baseado em APG II. second ed. Instituto Plantarum, Nova Odessa. [ Links ]
46. Theobald WL, Krahulik JL, Rollins, RC (1979). Trichome description and classification, in Metcalfe CR, Chalk L (eds), Anatomy of the Dicotyledons - systematic anatomy of the leaf and stem, Vol. I, 2nd ed. Oxford University Press, London, pp 40-53. [ Links ]
47. Wilkinson HP (1979). The plant surface (mainly leaf). Part I: stomata, in Metcalfe CR, Chalk L (eds), Anatomy of the Dicotyledons - systematic anatomy of the leaf and stem, Vol. I, 2nd ed. Oxford University Press, London, pp 97-117. [ Links ]
Received: September 9, 2009.
Revised version received: January 27, 2010.
Acepted: January 28, 2010.














