Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell vol.34 no.2 Mendoza mayo/ago. 2010
BRIEF NOTE
CD44 is involved in CXCL-12 induced acute myeloid leukemia HL-60 cell polarity
Liping Zhou*, Xiaolin Guo, Jing Ba, Lianshuang Zhao
Department of Laboratory Medicine, First Affiliated Hospital, China Medical University, NO.155, North Nanjing Street, Heping District, Shenyang 110001, China.
*Address correspondence to: Liping Zhou. E-mail: zhouliping750825@163.com
ABSTRACT: CXCL-12 and its receptor CXCR4 participate in breast cancer and melanoma cell metastasis to bone and lymphoid nodes. CD44, as a receptor for hyaluronic acid, is involved in lymphocyte recirculation, homing, adhesion and migration. But the role of CD44 in CXCL-12 induced leukemia cell migration still remains unclear. The present study showed that CXCL-12 stimulation induced the rapid internalization of CXCR4 and facilitated the formation of lamellipodia and uropod in acute leukemia cell line HL-60. CXCL-12 also induced CD44 translocation into the uropod, while CD44 remained evenly distributed on the untreated cell membranes. Results suggest that CD44 participates in CXCL-12 induced cell polarization and subsequent cell migration.
Key words: Cell migration; Lamellipodia formation; Uropod formation; Hyaluronic acid.
The chemokine stromal cell-derived factor-1 (CXCL-12, also named SDF-1) is the only powerful chemoattractant for human CD34+ progenitors (Aiuti et al., 1997). It is also one of the few chemokines that binds only to one receptor, CXCR4 (Burger and Kipps, 2006). CXCL12 is constitutivelyproduced in many organs including the human bone marrow, suggesting a major role for CXCL12 /CXCR4 interactions insteady-state homeostatic processes such as the control of leukocytetrafficking and retention of undifferentiated and maturing hematopoieticcells within the bone marrow in both normal and pathological conditions (Burger and Burkle, 2007; Gieryng and Bogunia-Kubik, 2007; Avigdor et al., 2004).
Adhesion molecule CD44 is a cell surface transmembrane glycoprotein encoded by a single gene. As a receptor for hyaluronic acid, CD44 is involved in lymphocyte activation, recirculation and homing, adhesion of extracellular matrix, angiogenesis, cell proliferation, cell differentiation and migration (Haynes etal., 1989; Slevin et al., 2007; Pals et al., 1993). CD44 and its down stream Rho-ROCK pathway is required for posterior protrusion, thereby the migration of cells (Lee et al., 2004; Bourguignon et al., 2003). CD44 is expressed on leukemic blasts in all acute myeloid leukemia subtypes (Wood, 2007; Gadhoum et al., 2004; Charrad et al., 2002), but the role of CD44 in these cells' chemotaxis and homing is still unclear. Here we show that CXCL-12 promoted lamellipodia formation of the human cell line HL-60 on hyaluronic acid, and CD44 was involved in cell polarization.
The human acute myeloid leukemia HL-60 cells were cultured in RPMI 1640 medium (Sigma, St. Louis, MO, USA) containing 10% fetal calf serum in 5% CO2 humidified air at 37°C. For flow cytometry assay, HL-60 cells were incubated with staining buffer (phosphate buffer-saline containing 0.03% bovine serum albumin) containing 4mg/ml antibodies as follows: fluorescein-conjugated anti-CXCR4 monoclonal antibody 2B11 (BD Bioscience, San Jose, CA, USA) and anti-CD44 antibody. The stained cells were washed and analyzed by flow cytometry (FACSCalibur, Becton-Dickinson).
For immunostaining, HL-60 cells were incubated in the presence or absence of 20 nM CXCL12 on hyaluronic acid-coated culture slides (Becton-Dickinson, San Jose, CA, USA) at 37°C for 30 min and then fixed with 3.3% paraformaldehyde for 15 min at room temperature, treated with 0.2% Triton X-100 for 2 min, and blocked with 5% bovine serum albumin for 30 min. Then the cells were exposed to anti-α-tubulin (Sigma, St. Louis, MO; dilution 1/400), or anti-CD44 monoclonal antibodies (BD Biosciences, USA; dilution 1/200) for 60 min. After washing, the cells were incubated with AlexaFluor488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA) and AlexaFluor594-conjugated phalloidin for 60 min. After washing, the stained cells were mounted with antifade-containing glycerol/phosphate buffer-saline and examined with a confocal laser-scanning microscope (TCS SP2, Leica, Germany).
As expected, HL-60 cells expressed both CXCL-12 and CD44 receptors (Fig. 1). Then the cells were stimulated with CXCL-12 and CXCR4 expression on the cell surface was detected by flow cytometry. The results showed CXCR4 began to internalize immediately and reached a baseline 5 min later, which was maintained for at least 30 more minutes (Fig. 2).
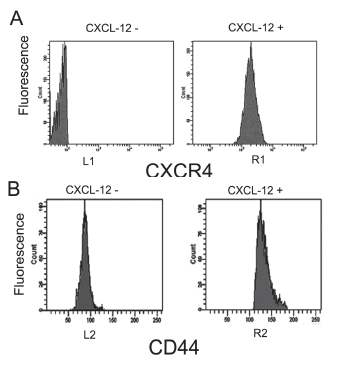
FIGURE 1. Expression of CXCR-4 and CD44 in HL-60 cells. Cells were incubated in 0.03% bovine serum albumin/RPMI containing fluorescein- conjugated anti-CXCR4 monoclonal antibody for 30 mins. Then the stained cells were washed and analyzed by flow cytometry. A. Expression of CXCR-4 in HL-60 cells. Cells were stained without (left panel) or with anti-CXCR4 antibody (right panel). B. Expression of CD44 in HL-60 cells. Cells were stained without (left panel) or with anti-CD44 antibody (right panel).
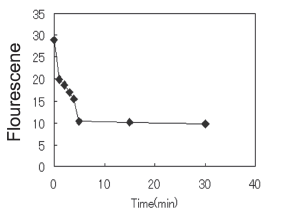
FIGURE 2. CXCR4 internalized by HL-60 cells after CXCL12 treatment. Cells were incubated in 0.03% bovine serum albumin/RPMI containing fluorescein-conjugated anti-CXCR4 monoclonal antibody for 30 mins on ice. Then they were stimulated with SDF-1, and the reaction was stopped by centrifuging at 4ºC at several times after stimulation. The stained cells were washed and analyzed by flow cytometry.
Also, HL-60 cells were treated with CXCL-12 for 15 mins, and lamellipodia and uropod formation was observed after immunofluorence staining (Fig. 3), suggesting that CXCL-12 can induce leukemia cell HL-60 migration.
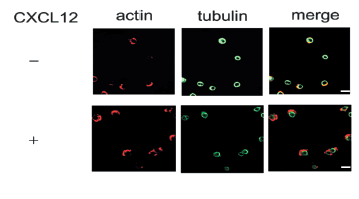
FIGURE 3. CXCL12 induced lamellipodia and uropod formation. HL-60 cells were incubated in the presence or absence of 20 nM CXCL12 on the HAcoated culture slides at 37ºC for 30 min. The cells were stained with anti-α-tubulin monoclonal antibody for 60 min, then followed by incubation with AlexaFluor488-conjugated goat anti-mouse IgG and AlexaFluor594-conjugated phalloidin for 60 min. Finally they were examined with a confocal laser-scanning microscope, and scale bars represent 10 μm.
Since Lee et al. (2004) have shown that CD44 participates in uropod formation in EL4.G8 T-lymphoma cells, we treated HL-60 cells with CXCL12 and investigated CD44 localization in them. Results showed that CD44 spread on the uropod after CXCL12 treatment, while it was evenly localized on the cell membrane of untreated cells (Fig. 4) suggesting that CD44 may play a role in uropod formation in HL-60 cells.

FIGURE 4. CXCL12 induced CD44 location on the uropod. HL-60 cells were incubated in the presence (upper panel) or absence (lower panel) of 20 nM CXCL12 on the hyaluronic acid-coated culture slides at 37ºC for 30 min. The cells were stained with anti-CD44 monoclonal antibody for 60 min, then followed by incubation with AlexaFluor488-conjugated goat anti-mouse IgG and AlexaFluor594-conjugated phalloidin for 60 min, and they were examined with a confocal laser-scanning microscope. Scale bars represent 10 μm. Arrows show uropod formation.
The present study showed that acute leukemia cell line HL-60 expressed both CXCR4 and CD44, and that CXCL-12 stimulation induced the rapid internalization of CXCR4 and facilitated the formation of lamellipodia and uropod in them. CXCL-12 also induced CD44 translocation into the uropod, while CD44 remained evenly distributed on the membrane of untreated cells. These results suggest that CD44 may have a significant role in CXCL-12 induced cell polarization and subsequent cell migration.
In recent years, a number of studies emphasized the idea that tumor cell migration and organ-specific metastasis are critically regulated by chemokines and their receptors and that. CXCL-12 participates as a multi-functional chemokine in this context (Karnoub and Weinberg, 2006-2007; Wang et al., 2006). CXCL-12 and its receptor CXCR4 participate in regulating the retention of normal hematopoietic stem cells within the bone marrow and their release into the circulation. They have also been shown to participate in breast cancer and melanoma cell metastasis to bone and lymphoid node (Tavor et al., 2004). In the current study, CXCL-12 stimulation induced the internalization of CXCR4 in HL-60 cells in a time-dependent manner. Also, after exposure to CXCL-12 for 15 mins, HL-60 cells formed lamellipodia and uropod formation, which also substantiates the possible role of the CXCL-12/CXCR4 axis in mediating HL-60 cell migration.
Two codependent steps have been proposed for promoting lymphocyte motile polarized morphology in response to chemoattraction, "posteriorization" of the plasma membrane mediated by ERM proteins phosphorylation, and "uropod protrusion", mediated by Rho-ROCK activation. pERM is important for CD44 binding to hyaluronan in myeloid cells (Brown et al., 2005). And our result showed CD44 localizated in the uropod, in a way similar to the report (Lee et al., 2004) that CD44 is concentrated in the uropod in CXCL12 treated lymphoma cells, suggesting that CD44 may be involved in the uropod formation induced by CXCL-12 in HL-60 cells.
In conclusion, CXCL12 stimulation induced HL-60 cells migration, and CD44 binding to hyaluronan and its concentration in the uropod may participate in uropod formation and cell migration.
References
1. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC (1997). The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to the peripheral blood. Journal of Experimental Medicine 185: 111-120. [ Links ]
2. Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T (2004). CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood 103: 2981-2989. [ Links ]
3. Burger JA, Kipps TJ (2006). CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107: 1761-1767. [ Links ]
4. Burger JA, Burkle A (2007). The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Bristish Journal of Haematology 137: 288-296. [ Links ]
5. Brown KL, Birkenhead D, Lai JC, Li L, Li R, Johnson P (2005). Regulation of hyaluronan binding by F-actin and colocalization of CD44 and phosphorylated ezrin/radixin/moesin (ERM) proteins in myeloid cells.Experimental Cell Research 303: 400-414. [ Links ]
6. Bourguignon LY, Singleton PA, Zhu H, Diedrich F (2003). Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. Journal of Biological Chemistry 278: 29420-29434. [ Links ]
7. Charrad RS, Gadhoum Z, Qi J, Glachant A, Allouche M, Jasmin C, Chomienne C, Smadja-Joffe F (2002). Effects of anti-CD44 monoclonal antibodies on differentiation and apoptosis of human myeloid leukemia cell lines. Blood 99: 290-299. [ Links ]
8. Gieryng A, Bogunia-Kubik K (2007). The role of the SDF-1-CXCR4 axis in hematopoiesis and the mobilization of hematopoietic stem cells to peripheral blood. Postpy higieny i medycyny do wiadczalnej 61: 369-383. [ Links ]
9. Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J, Robert-Lezenes J, Chomienne C, Smadja-Joffe F (2004). The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leukemia & Lymphoma 45:1 501-510. [ Links ]
10. Haynes BF, Telen MJ, Hale LP, Denning SM (1989). CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunology Today 10: 423-428. [ Links ]
11. Karnoub AE, Weinberg RA (2006-2007). Chemokine networks and breast cancer metastasis. Breast Disease 26: 75-85. [ Links ]
12. Lee JH, Katakai T, Hara T, Gonda H, Sugai M, Shimizu A (2004). Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. Journal of Cell Biology 167: 327-337. [ Links ]
13. Pals ST, Koopman G, Heider KH, Griffioen A, Adolf GR, Van den Berg F, Ponta H, Herrlich P, Horst E (1993). CD44 splice variants: expression during lymphocyte activation and tumor progression. Behring Institute Mitteilungen 92: 273-277. [ Links ]
14. Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S (2007). Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biology 26: 58-68. [ Links ]
15. Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, Lapidot T (2004). CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Research 64: 2817-2824. [ Links ]
16. Wood BL (2007). Myeloid malignancies: myelodysplastic syndromes, myeloproliferative disorders, and acute myeloid leukemia. Clinical & Laboratory Medicine 27: 551-575. [ Links ]
17. Wang J, Loberg R, Taichman RS (2006). The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Reviews 25: 573-87. [ Links ]
Received: July 11, 2008.
Revised version received: November 21, 2009.
Acepted: February 21, 2010.














