Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell vol.34 no.3 Mendoza sept./dic. 2010
ORIGINAL ARTICLES
Structure of the stigma and style in sunflower (Helianthus annuus L.)
M.M. Gotelli*, B.G. Galati, D. Medan
Grupo de Biología Reproductiva en Plantas Superiores, Cátedra de Botánica Agrícola, Facultad de Agronomía, Universidad de Buenos Aires. Av. San Martín 4453, (1417) Buenos Aires, Argentina.
Address correspondence to: Marina María Gotelli. E-mail: gotelli@agro.uba.ar
Abstract: This is the first report of the ultrastructure of the stigma and style during and after anthesis in Helianthus annuus L. using light and transmission electron microscopy. The stigma is bifid with unicellular papillae. There is no secretion of lipids, carbohydrates or proteins at anthesis. The style is semisolid in the upper portion, closer to the stigma, and becomes solid below. Ultrastructural changes on cells of the stigma and the style are described. The transmitting tissue of the ovule is first evident 40 minutes after pollination and persists during the first stages of embryogenesis. Only one pollen tube per micropyle was observed growing through this tissue.
Key words: Ultrastructure; Gynoecium; Ovule; Transmitting tissue.
Introduction
Raghavan (1997) considers that there is an imprecise correlation between the morphology of the stigmatic surface and the amount of secretion during the receptive period. Wet stigmas may have secretions primarily rich in lipids, as in Solanaceae, or rich in carbohydrates, as in Liliaceae (Goldman et al., 1994). Lipidic components seem to be essential for pollen tube penetration in the stigma and ulterior growth through the style (Wolters-Arts et al., 1998).
Dry stigmas, which lack a copious surface secretion, are covered by a continuous cuticle that must be penetrated enzymatically by pollen tubes (Heslop-Harrison et al., 1975), which is overlaid by a thin proteinaceous pellicle (Heslop-Harrison et al., 1975). In spite of the dry nature of cuticularized stigmas, a lipid surface is necessary for the hydration and germination of pollen grains (Wolters-Arts et al., 1998).
Heslop-Harrison and Shivanna (1977) described the stigmatic surface of the Asteraceae as dry, based on the observation of 17 species of different tribes. This description correlates with the sporophytic autoincompatibility system known for the Asteraceae (Hiscock, 2000a, b).
Knox (1973) showed that in Ambrosia sp.and in Cosmos bipinnatus compatible and incompatible pollinations where followed by a quick liberation of pollen wall material (pollenkitt) towards the stigmatic surface. Vithanage and Knox (1977) confirmed this in Helianthus and claimed that the stigmatic surface of this genus is dry. Elleman et al. (1992) re-examined pollination events in C. bipinnatus and Helianthus annuus and demonstrated that a stigmatic secretion was evidently induced by pollen-stigma contact. Therefore, Elleman et al. (1992) wondered if the stigmatic surface of the Asteraceae is entirely dry or if it is parcially secretory. Hiscock et al. (2002) studied pollen-stigma interactions in species of the Asteraceae, paying special attention to the nature of the stigmatic surface and its response to compatible and incompatible pollinations. Hiscock et al. (1998) describe the stigma of Senecio as semi-dry, since it is covered by a cuticle, a proteinaceous pellicle and a secretion of lipids, carbohydrates and proteins. These authors consider the semi-dry stigma to be a general trait of the family.
The ultrastructure of stigmatic papillae of some species is known (Heslop-Harrison and Heslop-Harrison, 1980; Cresti et al., 1986; Kandasamy et al., 1989; Bystedt, 1990) However, transmission electron microscopy studies of these cells on sunflower are lacking.
Several authors studied the ultrastructure of the transmitting tissue of Angiosperms (Johri, 1984; Raghavan, 1997), founding that the cells contain numerous mitochondria, plastids, rough endoplasmic reticule, dictyosomes and free ribosomes. The intercellular matrix of many solid styles is formed by carbohydrates, proteins, glycoproteins and some enzymes (Pandey, 1997). In sunflower, Yan et al. (1991) described the ultrastructure of the transmitting tissue of the ovule micropyle before and after fertilization claiming its close relationship between micropyle organization, orientation of pollen tube growth and synergid degeneration.
A complete understanding of the reproductive structures is of key importance for improvement of species with high economic value as sunflower. In a previous investigation a study of the development of female and male gametophyte was made (Gotelli et al., 2008). The aim of the present research is to study the complete pathway of pollen tubes in Helianthus annuus L. Since there are no published papers on the ultrastructure of the stigma and style of sunflower, we study these structures with transmission electron microscope before, during and after anthesis.
Materials and Methods
For light microscopy studies, flowers of the commercial hybrid P30, before, during and after anthesis were fixed in FAA (formalin, alcohol, acetic acid), dehydrated in an ethanol series, transferred to xylene and then embedded in paraffin wax. Longitudinal and transverse sections of 12 µm were cut following standard botanical microtechniques. The slides were stained with a safranin-fast green combination (D'Ambrogio, 1986). The material was viewed with a Wild M20 microscope and photographed with a Canon PowerShot A650 IS digital camera. Total proteins were localized with Coomassie Brilliant Blue (Heslop-Harrison et al., 1973), and lipids with Sudan Black B (Pearse, 1961).
For transmission electron microscopy, the stigmas and styles were pre-fixed overnight in 2.5% glutaraldehyde in phosphate buffer (pH 7.2) and then post-fixed in OsO4 at 2ºC in the same buffer for 3 h. Following dehydration in ethanol series, the material was embedded in Spurr's resin. Ultrathin sections (750 to 900 nm) were made on a Sorvall ultramicrotome and then stained with uranyl acetate and lead citrate (O'Brien and McCully, 1981). The sections were observed and photographed with a JEOL-JEM 1200 EX II TEM at 85.0 kV.
Results
The stigma has no conspicuous secretion at anthesis. Stigmatic papillae are unicellular with numerous nucleoli in each conspicuous nucleus. Before anthesis, these cells are highly vacuolated and have mitochondria, plastids with starch grains, abundant rough endoplasmic reticulum, numerous dictyosomes and some lipidic globules (Fig. 1). They present thick primary walls coated by a thin cuticle and a protein pellicle layer as revealed by staining with Sudan Black B and Coomassie Brilliant Blue, respectively. The protein pellicle can be distinguished from the cuticle as a thin layer of higher electron-density (Fig. 2). Many plasmodesmata are observed in the radial and inner tangential walls of these papillae (Figs. 3, 4). The wall is differentiated into two layers of similar thickness, the inner one with moderate electron-density and the outer one with a higher electron-density (Fig. 5).
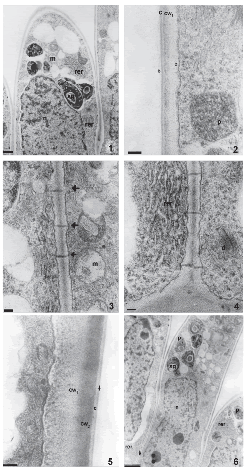
FIGURES 1-6. The stigma of Helianthus annuus (transmission electron micrographs) before (1-4) and during anthesis (5-6). (1) General aspect of papillae in longitudinal section, plastids (p) with starch grains (sg), mitochondria (m), nucleus and rough endoplasmic reticulum (rer). (2) Detail of the papillae wall with two different electron-densities (cw1, cw2), cuticle (c) and protein pellicle (arrow head), plastids (p) in the papilla. (3) Detail of plasmodesmata (arrows), mitochondria (m) (4) Abundant rough endosplamic reticulum (rer), dictyosome (d). (5) Detail of a papilla wall with two different electrodensities (cw1 y cw2), cuticle (c) and protein pellicle (arrow). (6) General aspect of the papillae, plastids (p) with starch grains (sg), rough endosplamic reticulum, nucleus (n) and plasmodesmata (arrow). Scale bars: 1: 1μm, 2-4, 5: 200 nm, 6: 2μm.
During anthesis, pollen grains start to germinate over the stigmatic papillae. Endoplasmic reticulum, dictyosomes, more mitochondria, lipidic globules and plastids with more starch grains are observed in these cells. At this stage, the two different layers of the papillae wall are more evident. Plasmodesmata that connect papillae with each other are still present (Fig. 6, arrow). The style consists of an epidermis, cortical parenchyma and a centrally-located transmitting tissue (Fig. 7). The style is semisolid. Both stylar branches are not completely fused, and a small opening in the middle of the transmitting tissue is left (Fig. 8).
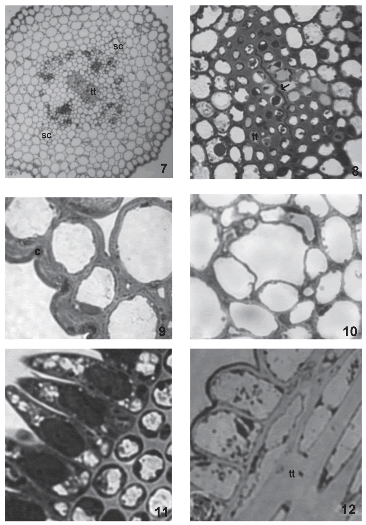
FIGURES 7-12. The style and stigma of Helianthus annuus (light micrographs). (7) General aspect of a transverse section of the style, two secretory channels (sc) and transmitting tissue (tt) (8) Transmitting tissue (tt) and union zone between both stylar branches (arrow). (9) Detail of the stylar epidermis with thick cuticle (c). (10) Secretory channel (sc) in the cortical parenchyma of the style. (11) Transversal section of the stigma. (12) Longitudinal section of the stigma, stigmatic papillae and detail of the transmitting tissue (tt). Scale bars: 7: 60μm; 8: 15μm; 9-10: 12μm; 11-12: 6μm.
The epidermis is formed by vacuolated cells with thickened outer tangential walls and a thick cuticle (Fig. 9). In the cortical parenchyma, two vascular periphloematic bundles and two schizogenous ducts (Fig. 10) can be distinguished. Parenchymatic cells possess thin walls and are highly vacuolated.
The transmitting tissue is placed subepidermically in the stigmatic papillae region (Figs. 11, 12) and centrally in the style. This tissue is conformed by isodiametric cells in transversal section (Fig. 11) and elongated, with sharp ends in longitudinal section (Fig. 12). Before anthesis, these cells have a more or less dense cytoplasm with abundant rough endoplasmic reticulum, dictyosomes with many vesicles surrounding them, and plastids with starch grains (Figs. 13, 14). The middle layer of the transmitting tissue cells is distended, particularly at the corners and shows moderate to low electron-density. The wall has a fibrilar appearance. Many plasmodesmata are observed between these cells and the papillae (Fig. 15).
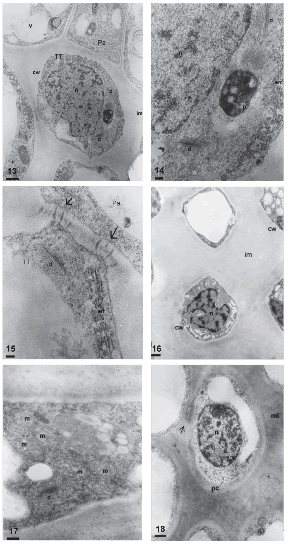
FIGURES 13-18. Transmitting tissue of Helianthus annuus before (13-15), during (16-17) and after anthesis (18) (transmission electron micrographs). (13) General aspect of the transmitting tissue (TT) in transversal section, cell wall (cw), nucleus (n), dictyosome (d), plastid (p), vacuole (v) intercellular matrix (im), papillae (Pa). (14) Detail of the cytoplasm of a cell of the transmitting tissue, plastids (p), rough endosplamic reticulum (rer), dictyosome (d), nucleus (n) (15) Contact zone between a papilla (Pa) and a cell of the transmitting tissue (TT), plasmodesmata (arrow), rough endosplamic reticulum (rer) y dictyosome (d). (16) Transmitting tissue, intercellular matrix (im), cell wall (cw) y nucleus (n). (17) Detail of the cytoplasm with numerous mitochondria (m) and dictyosome (d). (18) Transversal section of the transmitting tissue, cell wall (cw), intercellular matrix (im), nucleus (n) and rests of plasmodesmata (arrow). Scale bars: 13, 16, 18: 1μm; 14-15: 200nm; 17: 500nm.
During anthesis, the thin primary wall of the transmitting tissue cells is more clearly visualized since the intercellular matrix is still more distended than in the previous stage, with an amorphous appearance and low electron-density (Fig. 16). At this stage, many mitochondria and some dictyosomes are observed in the transmitting tissue (Fig. 17).
Once pollination takes place, pollen tubes grow through the middle lamella or the intercellular matrix of the transmitting tissue. Transmitting tissue cells are observed highly vacuolated, with the cytoplasm confined to cell periphery, showing few plastids and lipidic globules. The intercellular matrix at post-anthesis is more electron-dense and less distended than in previous stages (Fig. 18).
In the micropylar area, the integument has more cellular layers at the side next to the funicle. During anthesis, the integument cells delimiting the funicular side of the micropylar canal show an intercellular matrix that reacts to colorants similarly as the stylar transmitting tissue. This ovular transmitting tissue can be first observed 40 minutes after pollination and persists during the first stages of embryogenesis (Figs. 19-21). Only one pollen tube per micropyle was observed growing through the ovular transmitting tissue.
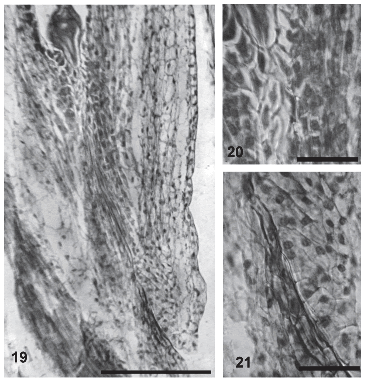
FIGURES 19-21. Transmitting tissue in the ovule of Helianthus annuus (light micrographs). (19) General aspect of the transmitting tissue in the funicular side (arrow). (20) Detail of the transmitting tissue in the micropylar end of the female gametophyte (arrow). (21) Detail of the transmitting tissue in the micropylar end of the ovule (arrow). Scale bar: 19: 60μm; 20-21: 30μm.
Discussion
The stigma of Helianthus annuus does not show signs of a copious secretion. Therefore, it belongs to the dry type, which confirms previous reports for sunflower (Vithanage and Knox, 1977) and other species of the family Asteraceae (Heslop-Harrison and Shivanna, 1977). Elleman et al. (1992) found a secretory response of the stigma after pollination in Cosmos bipinnatus and Helianthus annuus and wondered if the stigmatic surface if the Asteraceae is entirely dry or partially secretory. Hiscock et al. (2002) describe the stigma of Senecio squalidus (Asteraceae) as semi-dry and anticipate that it may be a trait common to the family. The presence of the secretion formed by lipids, carbohydrates and proteins that the authors mention could not be confirmed because field observations or tests in fresh material were not made at the anthesis stage. However, it could be observed that the cuticle of stigmatic papillae in sunflower is just overlaid by a protein pellicle, as is reported for other species with dry stigmas (Heslop-Harrison and Heslop-Harrison, 1980; Heslop-Harrison et al., 1984; Hiscock et al., 1998).
The nature of the stigmatic papillae wall is very diverse. According to Heslop-Harrison and Heslop-Harrison (1980) protein vesicles may be included in this wall. In sunflower, like in Secale cereale (Heslop-Harrison and Heslop-Harrison, 1980) and Zea mays (Heslop-Harrison et al., 1984), the papillar wall is differentiated in two layers with different electron-density and vesicle inclusions in it were not observed.
Before anthesis, great amount of endoplasmic reticulum was observed in the stigmatic papillae of sunflower. According to Dumas (1973), this organelle is related to the formation of secretory vesicles with lipophilic fluid. In sunflower, the presence of reticulum at this stage could be directly related to the secretion of the protein pellicle.
Transmitting tissue cells of sunflower have a peripheral cytoplasm with some mitochondria, numerous plastids with starch grains, dictyosomes and several lipidic globules. According to Raghavan (1997), cells with abundant ribosomes, mitochondria, endoplasmic reticulum, dictyosomes and amyloplasts are metabolically very active.
The intercellular matrix observed for this and other species is believed to be of an amorphous nature, with a mucilaginous base, carbohydrates, proteins, phenolic compounds and tannins (Raghavan, 1997). This mucilaginous matrix allows pollen tubes to grow and guides them (Clarke et al., 1977).
Yan et al. (1991) studied the ultrastructure of the sunflower micropyle in relation to pollen tube growth and the degeneration of one of the synergids of the megagametophyte. These authors mention the presence of a transmitting tissue in the micropyle produced by the epidermic and subepidermic cells of the integument. According to the observations made in this work, this tissue is formed by the epidermis and one or two subepidermic layers of the integument that limit the micropyle. Yan et al. (op cit.) describe integument cells adjacent to the micropyle, next to the funicle, as similar to the ones of the transmitting tissue that constitutes an optimal environment for pollen tube growth. These authors claim that more than one pollen tube may grow through this tissue. However, we observed only one pollen tube per ovule.
This first report of the ultrastructure of the stigma and style during and after anthesis in Helianthus annuus L. will be completed with a temporal and detailed description of all events from the pollen grain arrival to the stigma until the mature embryo is formed.
References
1. Bystedt PA (1990). The transmitting tract in Timezia fosteriana (Iridaceae). I. Ultrastructure in the stigma, style and ovary. Nordic Journal of Botany 9: 507-518. [ Links ]
2. Clarke AE, Considine JA, Ward R, Knox RB (1977). Mechanism of pollination in Gladiolus: roles of the stigma and pollentube guide. Annals of Botany 41: 15-20. [ Links ]
3. Cresti M, Ciampolini F, Tiezzi A (1986). Ultrastructural studies on Nicotiana tabacum pollen tubes grown in different culture medium (preliminar results). Acta Botanica Neerlandica 35: 285-292. [ Links ]
4. D'Ambrogio A (1986). Manual de técnicas en histología vegetal. Hemisferio Sur S.A. Buenos Aires, Argentina. [ Links ]
5. Dumas C (1973). Contribution à l'étude cyto-physiologique du stigmate III. Evolution et rôle du réticulum endoplasmique au cours de la sécrétion chez Forsythia intermedia Z.; Étude cytochimique. Zeitschrift für Pflanzenphysiologie 70: 119-130. [ Links ]
6. Elleman CJ, Franklin-Tong VE, Dickinson HG (1992). Pollination in species with dry stigma: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytology 121: 414-424. [ Links ]
7. Goldman MHS, Goldberg RB, Mariani C (1994). Female sterile tobacco plants are produced by stigma specific cell ablation. EMBO Journal 13: 2976-2984. [ Links ]
8. Gotelli MM, Galati B, Medan D (2008). Embryology of sunflower (Helianthus annuus L.). Annales Botanici Fennici 45:81-96 [ Links ]
9. Heslop-Harrison J, Heslop-Harrison Y (1980). The pollen-stigma interaction in grasses. I. Fine structure and cytochemical of the stigmas of Hordeum and Secale. Acta Botanica Neerlandica 29: 261-276. [ Links ]
10. Heslop-Harrison J, Heslop-Harrison Y, Knox RB, Howlett B (1973). Pollen wall protein: gametophytic and sporophytic fraction in the pollen wall of the Malvaceae. Annales of Botany 37: 403-412. [ Links ]
11. Heslop-Harrison J, Heslop-Harrison Y, Barber J (1975). The stigma surface in incompatibility responses. Proceedings of the Royal Society London B. 188: 287-297. [ Links ]
12. Heslop-Harrison Y, Shivanna KR (1977). The receptive surface of the angiosperm stigma. Annals of Botany: 1233-1258. [ Links ]
13. Heslop-Harrison Y, Reger B J, Heslop-Harrison J (1984). The pollen -stigma interaction in the grasses. 5. Tissue organization and cytochemistry of the stigma ("silk") of Zea mays L. Acta Botanica Neerlandica. 33: 81-99. [ Links ]
14. Hiscock SJ (2000a). Genetic control of self incompatibility in Senecio squalidus L. (Asteraceae)- a succesful colonising species. Heredity 84: 10-19. [ Links ]
15. Hiscock SJ (2000b). Self incompatibility in Senecio squalidus L. (Asteraceae). Annals of Botany 85 (suppl A): 181-190. [ Links ]
16. Hiscock SJ, Doughty J, Dickinson HG (1998). Unilateral incompatibility and the S (self-incompatibility) locus. In: Reproductive biology in systematics, conservation and economic botany (S Owens, PJ Rudall, eds.), p. 31-46. Royal Botanic Gardens, Kew. [ Links ]
17. Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG (2002). The stigma surface and pollen-stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. International Journal of Plant Science 163: 1-16. [ Links ]
18. Johri BM (1984). Embryology of Angiosperms. ed. Springer- Verlang- Berlin- Herdelberg-New York-Tokyo. [ Links ]
19. Kandasamy MK, Paolillo DJ, Faraday CD, Nasrallah JB, Nasrallah ME (1989). The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Develomental Biology. 134: 462-472. [ Links ]
20. Knox RB (1973). Pollen-wall proteins: pollen-stigma interactions in ragweed and Cosmos (Compositae). Journal of Cell Science. 12: 421-443. [ Links ]
21. O'Brien TP, McCully ME (1981) The study of plant structure. Principles and selected methods. Termarcarphi Pty. Ltd.: Melbourne, Australia. [ Links ]
22. Pandey AK (1997). Introduction to the embryology of Angiosperms. CBS Publishers and Distributors. Daryaganj, New Delhi, India. [ Links ]
23. Pearse AGE (1961). Histochemistry, theoretical and applied. 2nd ed. Boston, Little Brown. [ Links ]
24. Raghavan V (1997). Molecular Embryology of Flowering Plants. Cambridge. University Press. [ Links ]
25. Vithanage HIMV, Knox RB (1977). Development and cytochemistry of stigma surface and response to self and foreign pollination in Helianthus annuus. Phytomorphology 27: 168-179. [ Links ]
26. Wolters-Arts M, Lush WM, Mariani C (1998). Lipids are required for directional pollen-tube growth. Nature 392: 818-821. [ Links ]
27. Yan H, Yang H-Y, Jensen W (1991). Ultrastructure of the micropyle and its relationship to pollen tube growth and synergid degeneration in sunflower. Sexual Plant Reproduction 4: 166-175. [ Links ]
Received: March 18, 2010.
Revised version received: September 20, 2010.
Acepted: November 23, 2010.














