Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell vol.35 no.3 Mendoza sept./dic. 2011
ORIGINAL ARTICLES
Apigenin inhibits cell migration through MAPK pathways in human bladder smooth muscle cells
Qingxin Liu1§ , Xianggui Chen2§, Guolin Yang1, Xuewen Min3, and Maoxian Deng1,*
1. Department of Animal Biology, School of Husbandry and Veterinary Medicine, Jiangsu Polytechnic College of Agriculture and Forestry, Jurong, Jiangsu 212400, China
2. School of Bioengineering, Xihua University, Chengdu, Sichuan 610039, China
3. Department of Pathology, People's Hospital of Jurong, Jurong, Jiangsu 212400, China
§ These authors contributed equally to this work.
*Address correspondence to:
Maoxian Deng.
Department of Animal Biology, School of Husbandry and Veterinary Medicine, Jiangsu Polytechnic College of Agriculture and Forestry, 3 Changjiang Road, Jurong, Jiangsu 212400, P.R.China. Fax: (+86-511) 87291036. E-mail: dengok@hotmail.com
ABSTRACT: Apigenin, a nonmutagenic flavonoid, has been shown to possess free radical scavenging activities, anticarcinogenic properties, antioxidant and anti-inflammatory effects. Recently, apigenin was reported to cause gastric relaxation in murine. To assess possible effects of apigenin on migration of bladder smooth muscle (SM) cell, we isolated SM cells from peri-cancer tissue of human bladder and established a cell model that was capable to overexpress transiently MEKK1 (MEK kinase 1). Results showed that overexpression of active human MEKK1 by adenoviruses infection induced migration of human bladder smooth muscle (hBSM) cells and phosphorylation of MAPKs, ERK, JNK and p38, which are the downstream molecules of MEKK1. Then, hBSM cell overexpressing MEKK1 were exposed to apigenin (50 μM). Our data indicated that apigenin inhibited significantly activation/phosphorylation of MAPKs and migration of hBSM cells induced by MEKK1 overexpression. Besides, apigenin inhibited actin polymerization, which underlines muscle contraction and cell migration. The results suggest that apigenin inhibits activation of MAPKs and thereby the cell migration. The mechanism might be that apigenin blocks signal transmission from MEKK1 to MAPKs.
Keywords: Flavonoid; Cell motility; MAP kinase
Introduction
Flavonoids comprise a vast array of polyphenolic compounds ubiquitous in plants, many of which have been used in Traditional Chinese Medicine for thousands of years. Apigenin, a polyphenolic bioflavone (4,5,7-trihydroxyflavone), is a nonmutagenic chemopreventive agent found in a variety of Chinese herbs and green leafy vegetables, especially in parsley, thyme, peppermint, olives and herbs. Recently, apigenin has been reported to exhibit a wide range of biological effects on animal and human health (Li et al., 2009; Chuang et al., 2009; Jeong et al., 2009). It is described to inhibit markedly the proliferation of cancer cells (Yin et al., 2001, 1999), the growth of leiomyomal smooth muscle cells (Kim et al., 2005) and the migration of endothelial and tumor cells (Kim et al., 2011; Noh et al., 2010; Franzen et al., 2009; Zou and Chiou, 2006). In addition, apigenin has been reported to modulate vascular tone (Ajay et al., 2003; Morello et al., 2006; Rotondo et al., 2009) and depress contraction of smooth muscle (Gharzouli and Holzer, 2004; Yousufzai et al., 2000) and vas deferens (Capasso et al., 2006).
Cell migration is critical and highly regulated for many physiological and pathological processes, such as angiogenesis, immune response, inflammation, development, wound healing, tumor progression and metastasis. Smooth muscle migration participates in various processes, including regulation of blood pressure, gastric peristalsis, bladder emptying and airway spasm. A huge variety of intracellular and extracellular signaling molecules have been implicated in cell migration. Cells are able to respond to extracellular stimuli such as mitogens and hormones, and convert these signals into cellular processes. Thousands of signal molecules form highly interactive networks to achieve the integrated function of cells in an organism. In the intracellular signaling networks, one of the most fundamental pathways is the mitogen-activated protein kinase (MAPK) cascades. The previous study shows that MAP kinase pathway plays a role in keratinocytes migration (Zhang et al., 2005). The increasing evidences have shown the inhibitory effect of apigenin on phosphorylation of MAPKs, such as ERK (Mounho and Thrall, 1999; Kim et al., 2002; Yin et al., 2001) and p38 (Van Dross et al., 2003; Long et al., 2008).
Although studies have being shown that apigenin may affect SM cell growth and muscle contraction, these still remain controversial. The related mechanisms have not yet been elucidated. In this manuscript, we investigated the effects of apigenin on cell migration using MEKK1-overexpressing primary hBSM cells as a biological model. To elucidate further the mechanisms by which apigenin inhibits smooth muscle cell migration, we extended our investigation to effects of apigenin on MEK/MAPKs pathways. Here, we report that apigenin inhibits MAPKs' activation and hBSM cell migration induced by MEKK1 overexpression.
Materials and methods
Reagents and antibodies
Apigenin-(4,5,7-trihy-droxyflavone) was purchased from Sigma, MO, USA. This phytocompound was prepared in dimethyl sulfoxide (DMSO) at 10 mg/ml as stock solutions and added to the culture media to achieve the desired final concentration. The final volume of solvent did not exceed 0.5% of media. According to preliminary test, we chose a final apigenin concentration of 50 μM for all subsequent experiments.
Antibodies against phosphor-JNK(1/2) purchased from Sigma, MO, USA. Anti-phospho-p38 (Thr-180/Tyr-182) and anti-phosphou-ERK(Tyr-204) antibodies were obtained from New England Biolabs, Inc. (Beverly, MA, USA). Anti-ERK1/2 antibody was purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Antibodies against smooth muscle actin, h-caldesmon, HA and JNK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody for smooth muscle myosin heavy chain was product of Abcam (Cambridge, MA,USA).
Cell isolation and culture
Human bladder smooth muscle cells (hBSM) were isolated from peri-cancer tissue of a bladder cancer patient. The fresh bladder tissues were washed with pre-cooled PBS for twice. A piece of tissue (~1 gram) was cut and kept at -70ºC for Western blotting. After removal of the mucosa and serosa, bladder tissue was minced into small pieces (1 ∞ 1 mm) with sterilized scissors. To dissociate bladder myocytes, the tissue was digested with collagenase IV (1 mg/ml; Invitrogen) solved in M199 medium (Invitrogen, Carlsbad, CA, USA) in a slowly shaking water bath at 37ºC for 3 hours. Digested tissue was passed through a cell filter strainer. The cells were collected by centrifugation at 1,000 g at 4ºC for 10 min. Then, the pellet was washed with M199 medium for five times and finally resuspended and cultured in M199 supplemented with 10% fetal calf serum in humidified atmosphere of 5% CO2-95% air at 37ºC.
Cells were passaged by brief treatment with trypsin (0.25% trypsin-EDTA; Invitrogen, Carlsbad, CA, USA). The main biological characteristics of the cells were determined by Western blotting. Cells were regularly seeded into 10-cm plates with medium changed every other day. For experiments, when grown to 80% confluence, cells were digested with 0.25% trypsin-EDTA and plated in 6- and 24-well plates. For serum starvation, cells were washed twice with warm PBS and kept in serum-free M199 for overnight. Cells that were passaged less than seven times were used for experiments.
Virus production and infection of hBSM cells
To generate adenoviruses to express MEKK1, a HA-tagged 4.9-kb DNA fragment containing the cDNA for human wild-type MEKK1 was inserted to downstream of cytomegalovirus (CMV) promoter of p-shuttle vector (Stratagene Co., La Jolla, CA,USA) with GFP (green fluorescence protein) expression frame. Then, the MEKK1-containing shuttle vector was recombined with adenoviral DNA in bacteria to prepare MEKK1-bearing viral DNA. The recombinant adenoviral plasmid was packaged in packaging cells to produce adenovirus as described previously (He et al., 1998). Control adenovirus was prepared in the same procedure but no interesting gene was ligated to plasmid. After amplification, viruses were titrated by agarose overlay plaque assay.
Viral infection was carried out by incubating adenoviruses with hBSM cells at 20 PFU (plaque-forming unit) per cell for 1 h. In brief, M199 medium was replaced by 1 ml for 3.5-cm plates or 4 ml for 10-cm plates with fresh medium. Adenoviruses were added onto cells directly and mixed. Dishes were kept in CO2 culture incubator and rocked once every 15 minutes. After one hour, excess virus was removed and growth medium was supplied to normal level. After viral infection, cells were grown for 72 h before harvesting for Western blotting or immunostaining (except for special indication).
Immunoblotting and immunostaining
Frozen tissue was homogenized in ice-cold lysis buffer by a pre-cooled glass homogenizer. The lysis buffer consisted of 50mM Tris, 300mM NaCl, 3 mM EGTA, 0.1mM sodium orthovanadate, 10% glycerol v/v, 1% NP-40 v/v, and 0.3% SDS w/v, pH 7.6. Protease and phosphatase inhibitor cocktails (Sigma Co., Germany) were added before use (except for special indication).
The hBSM cells were harvested at indicated timepoints. In brief, dishes were put on ice and medium was removed. Cells on dishes were washed with ice-cold 1∞ PBS and then the lysis buffer was added onto cells. Immediately, cells were scraped into pre-cooled 1.5 ml centrifuge tubes by cell scrapers. The cells and homogenized tissue with lysis buffer were rotated at 4ºC for 30 minutes and then spun down at 14,000 rpm at 4ºC for 10 minutes. Total protein concentration in supernatant was measured with Pierce Protein Assay Reagents. Protein extracts were loaded onto 8% PAGE-SDS gels, which was subjected to electrophoresis in running buffer (BioRad Co., Hercules, CA, USA). Proteins were transferred overnight to nitrocellulose membrane by semi-dry transfer machine. After blocked in 5% milk in TBST buffer (25mM Tris, 140mM NaCl, 3mM KCl, pH7.4 and 0.1% Tween 20 v/v was added before use) for one hour, membranes were incubated with specific primary antibodies plus actin antibody as a loading control for one-hour shake at room temperature or overnight at 4ºC. Membranes were washed 3 times (5 minutes each) by TBST buffer, followed by one-hour incubation with secondary antibodies at room temperature. After 3-time wash in TBST, membranes were developed by ECL Western Blotting Detection Reagents (GE Healthcare, NJ, USA).
For immunostaining, cells grown on cover glasses were fixed (4% paraformaldehyde), permeabilized (0.5% Tween 20), and stained with first antibody at 37ºC for 1 h in wet box, followed by incubated with the appropriate fluorescence-labeled second antibody with FITC for 30 min. Cell nucleoli was stained with DAPI. The staining was visualized by using a fluorescence microscope. The Western blot bands were scanned and measured for density using the Image-Pro Plus 5.0 software.
Cell migration assay
For cell migration assay, 70% confluent monolayers of hBSM cells in 24-well collagen-coated plates were infected with adenoviruses at 20 PFU/cell for 1 h. Twenty four hours later, the infected cells were subjected to starvation in the absence of growth factors and serum for overnight. When cell density reached up to 90%, scratch wounds were created on the cell surface with a micropipette tip. The hBSM cell migration in scratch wounds was photographed at indicated timepoints.
For non-infected migration assay, 90% confluent monolayers of hBSM cells were scratched in the middle of wells to create wounds. Then, cells were washed twice immediately with and kept in serum-free medium containing 50 μM apigenin until cell migration was photographed. Movement of cells into the wounded areas, i.e. empty areas, was quantified using an Image-Pro plus 5.0. Cell migration was expressed as reduction of the areas.
F-actin staining
To stain F-actin (polymerized actin), cells grown on collagen-coated coverslips were washed twice with prewarmed PBS (pH 7.4) followed by fixing in 4% paraformaldehyde solution in PBS for 10 minutes at room temperature. After two-time washing with PBS, cells were permeabilized with prewarmed 0.1% Triton X-100 in PBS for 1 min. Then, cells were washed for twice and incubated in Rhodamine-phalloidin diluted 1:100 in PBS for 15 min. Finally, cells were rinsed for three times in PBS, 5 min/wash. Highest quality digital TIF images were obtained by a Nikon microscope, and the intensity and area of fluorescence of phalloidin staining of microfilaments were quantitively measured using the Image Pro Plus 5.0 software (Media Cybernetics, Inc, Silver Spring, MD, USA). The images at a magnification were imported into the Image-Pro Plus software, where they were calibrated to a known area of measurement. The phalloidin stain was then selected using the "color selection" function and the "area/density (intensity) measurement" functions were used to calculate the respective values. The maximal value was set to 100.
Results
Cultured hBSM cells possessed main biochemical characteristics of hBSM
Smooth muscle myosin heavy chain (SMMH), h-caldesmon (h-CaD) and actin (SMA) are considered specific markers of smooth muscle. To quantify the percentage of myocytes in isolated cells and expression of these markers of smooth muscle in cells, a preliminary assay for expression levels of SMMH, h-CaD and SMA among bladder tissue, cells of second and sixth generations was performed with Western blotting. The equal amounts of total protein (25 μg) from homogenized bladder tissue and cells were loaded onto 8% SDS-PAGE gel and separated by electrophoresis. After transferred to nitrocellulose membrane at 4ºC for overnight, proteins were detected with specific antibodies as described in Materials and methods. The results show that myocytes of two and six generation expressed SMMH, h-CaD and SMA (Fig. 1A). There was no significant difference in expression of SMMH, h-CaD and SMA between tissue and the cultured cells (2nd and 6th generation). Besides, distribution of smooth muscle actin in cell was visualized by immunostaining of SMA antibody (showed in Fig.1 B).
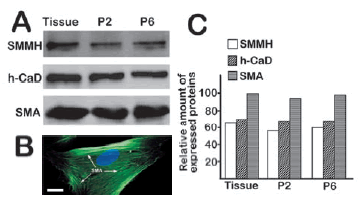
Figure 1. Expression of SMMH, h-CaD and SMA in bladder tissue and isolated myocytes. Equal amounts (25 µg) of protein extracts from tissue and cells were loaded onto 8% SDS-PAGE gel for Western blotting as described in Materials and methods. In addition, SMA in cultured cells was visualized following immunostaining procedure. (A) SMMH, h-CaD and SMA expression in bladder tissue, cultured cells of second generation (Line P2) and sixth generation (Line P6). (B) SMA fibers in isolated myocytes.
Adenovirus infected hBSM cells and expressed MEKK1at high efficiency
Infection efficiency of adenovirus depends on viral activity and varies with types of tissues or cells. To observe infection efficiency of the adenovirus on target cells, a green fluorescent protein (GFP) gene has already been inserted to viral DNA through a shuttle vector. Thus, GFP expressed in live cells can be a marker for viral infection and gene expression efficiency. According to the percentage of fluorescent cells, the infection efficiency was higher than 95% (shown in Fig. 2A).

Figure 2. Efficiency of viral infection and gene expression of MEKK1 in hBSM cells. (A) Exogenous GFP expression in hBSM cells 72 hours after viral infection. (B) Overexpression of MEKK1 in hBSM cells. Adenovirus-infected hBSM cells were harvested for Western blotting 72 hours after viral infection. HA-MEKK1 fusion protein was detected by antibody against HA. adenoviruses at 20 PFU/cell for 1 h. Twenty four hours later, the infected cells were subjected to starvation in the absence of growth factors and serum for overnight. When cell density reached up to 90%, scratch wounds were created on the cell surface with a micropipette tip. The hBSM cell migration in scratch wounds was photographed at indicated timepoints.
A HA tag has preliminarily been ligated to the upstream of MEKK1 cDNA to form HA-MEKK1 fusion protein. Therefore, amount of HA product stands for the expression level of MEKK1. Three days after viral infection, cells were harvested to evaluate the efficiency of MEKK1 expression. Cell lysates were loaded onto 8% SDS PAGE gels for Western blotting. MEKK1 was probed with antibody against HA tag. The Western blotting assay shows that adenovirus expressed MEKK1 at high level in myocytes infected with MEKK1-containing virus (Fig. 2B).
Apigenin inhibited hBSM cell migration induced by overexpression of MEKK1
Cell migration is a complex and highly coordinated fundamental process required during tissue formation, wound healing, immune surveillance, inflammatory response, and tumor metastasis (Lauffenburger and Horwitz, 1996). In smooth muscle, cell migration contributes significantly to physiological and pathological hyperplasia of vasculature, airway and detrusor urinae muscle. Under physiological conditions, cells are continuously and often simultaneously exposed to a variety of extracellular signals to which they must mount rapid and appropriate responses. That is to say, when an exogenous gene is introduced to cells, they make corresponding response.
To assess the possible effects of MEKK1 for smooth muscle cell motility, we infected growing hBSM cells with adenovirus containing active human MEKK1. Twenty tour hours later after adenovirus infection, a scratch wound was created in the middle of wells. Cell motility was photographed at indicated timepoints. Cell migration assay shows that MEKK1 overexpression induced hBSM cell migration. To evaluate effects of apigenin on migration of hBSM cells induced by MEKK1, cells were exposed to 50 μM apigenin immediately after a wound was created. Then, cell movement was photographed at indicated timepoints. The results show that apigenin inhibited motility of hBSM cells (Fig. 3).

Figure 3. Apigenin inhibited hBSM cell migration induced by adenovirus-mediated overexpression of MEKK1. Twenty four hours after viral infection, a scratch was made with a micropipette tip. Cells were exposed to apigenin (50 µM) immediately. Cell motility was photographed at 0, 8 and 20 hours later. Compared with the untreated cells (A) and control virus-infected cells that expressed only GFP (B), the cells infected with MEKK1-containing virus moved and covered the gap completely at 20 hours (C). Addition of apigenin at 50 µM onto MEKK1-overexpressing cells inhibited the migration induced by MEKK1 (D).
Apigenin inhibits phosphorylation of MAPKs induced by MEKK1
To investigate further the mechanism by which apigenin suppresses MAPK-mediated cell migration, we examined the effects of apigenin on activation of MAPKs in MEKK1-overexpressing cells. When cell density reached up to 70%, hBSM cells were infected with MEKK1-containing adenovirus at 20 PFU/cell. Forty eight hours later after viral infection, infected hBSM cells were exposed to apigenin of 50 μM in M199 medium for overnight. Then, cells were harvested and cell lysate was subjected to Western blotting as described in Materials and methods. The membranes were probed with antibodies against total MAPKs and phospho-MAPKs separately. As shown in Figure 4, apigenin prevented phosphorylation of ERK, JNK and p38 while overexpression of active MEKK1 induced their phosphorylation. These data suggest that apigenin blocks activation of MAPKs by MEKK1and thereby cell migration.
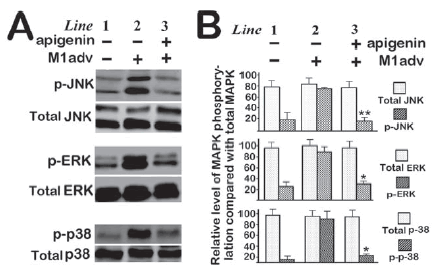
Figure 4. Apigenin inhibited MEKK1-induced phosphorylation. Two days after viral infection (20 PFU/cell), hBSM cells were incubated with apigenin (50 µM) for overnight. Then, cells were harvested for Western blotting as described in Materials and methods. Total MAPKs and phosphor MAPKs were detected by pan- antibodies and specific phosphor antibodies, respectively.
Apigenin inhibited actin polymerization
Actin filaments are one of important stress fibers, which consist of actin filaments, crosslinking proteins (such as caldesmon) and myosin II motors. Stress fibers are contractile cytoskeletal structures that provide cell with force for contraction and motility. Based on current understanding, the cell motility is driven by the propulsive force developed by the polymerization actin filaments. To understand the detailed mechanism by which apigenin inhibits cell motility, we investigated its possible effects on actin polymerization in hBSM cells by exposing untreated and MEKK1-expressing cells to apigenin. Then, polymerized actin was stained. The results suggest that apigenin depressed formation of F-actin in most cells (Fig. 5). However, we did not observe markedly difference of actin polymerization between the untreated cells and the cells infected with active MEKK1-containing adenovirus. The reason is that actin is likely already polymerized in adhering cells.

Figure 5. Effects of apigenin on actin polymerization. Cells grown on coverslips were fixed with 4% paraformaldehyde followed by permeabilization with 0.1% Triton X-100. Cells were stained with Rhodamine-phalloidin.
Discussion
The main finding of this study is that apigenin inhibits MEKK1-induced migration of hBSM cell through preventing MAPK phosphorylation.
Cell migration underlines a number of physiological and pathological processes in many different cell types, including wound healing, embryonic development, immune response, and tumor metastasis. In smooth muscle, cell migration contributes significantly to physiological and pathological hyperplasia of the vasculature, urinary tract and the airway. Bladder smooth muscle cells are recruited during bladder contraction and contribute to the related pathological processes. In the past several decades tremendous progress has been made in elucidating the mechanism of cell migration and regulation. However, the task for the understanding of these mechanisms is significantly complicated and hard. In recent years, there are a lot of reports indicating that flavonoids possess multiple biological effects, including inhibition for cell migration and contraction (Kim et al., 2011; Noh et al., 2010; Franzen et al., 2009; Morello et al., 2006; Rotondo et al., 2009).
Although studies show that apigenin exhibits effects on cell movement-related processes, including metastasis of a variety of types of cancer, the results are controversial. Furthermore, the specific molecular mechanisms behind the chemopreventive effects remains largely unelucidated (Hu et al., 2008; Lee et al., 2008; Lamy et al., 2008). In this study, we first isolated myocytes and examined the expression of smooth muscle myosin heavy chain (SMMH), h-CaD and SMA in cells. The isolated cells possessed main biochemical characteristics of smooth muscle. Then, we confirmed inhibitive effect of apigenin for cell migration in the primary human bladder smooth muscle cells.
Effects of apigenin on cell motility have being confirmed by increasing reports but the mechanism remains largely unknown.A recent report shows that apigenin may surpress the mogration of tumor cell through inhibiting FAK/Src signaling. When the experiments were finished, a paper reported apigenin inhibited the motility of cancer cell through suppressing the p38 MAPK signaling pathway (Noh et al., 2010) and Janus kinase 3 (Henkels et al., 2010). To investigate further the possible mechanism by which apigenin suppresses SM cell movement, we examined the effect of apigenin on activation of MAPK pathway. MAPKs are considered a regulator of cell migration since they can activate myosin light chain kinase, which induces serine phosphorylation of the myosin II regulatory light chain and thereby initiates cell movement (Klemke et al., 1997; Nguyen et al., 1999).
A canonical MAPK module generally consists of three levels of protein kinases: MAPKKKs (mitogen-activated protein kinase kinase kinases), MAPKKs (MEKs) and MAPKs. The first enzyme in the module is a MAPKKK enzyme, of which MEKK1 and Raf are the most important molecules. The MAPKKK enzymes are Ser/Thr protein kinases that activate the MEK enzymes, the second level of signal molecules, by phosphorylating two serine or threonine residues within a Ser-X-X-X-Ser/Thr motif. The MEKs include MEK1, 2, 3, 4 and 6 that are activated by the upstream MAPKKK enzymes and transmit signals to the downstream enzymes, MAP kinases. Once phosphorylated, MEK enzymes activate MAPKs by dual phosphorylation on Thr and Tyr within motif Thr-Glu-Tyr (ERK), Thr-Pro-Tyr (JNK) or Thr-Gly-Tyr (p38). MAPKs, the third level of molecules comprise three major enzymes, extracellular signal-regulated kinase (ERK), C-Jun N-terminal kinase (JNK) and p38.
ERK, JNK and p38 have been shown to regulate cell migration (Rousseau et al., 1997, 2006; Hedges et al., 1999; Zeigler et al., 1999; Xia and Karin, 2004). Activation of these MAPKs results in phosphorylation of many proteins with substantial regulatory functions throughout the cell, including other protein kinases, transcription factors, cytoskeletal proteins and other enzymes.
The increasing evidences have suggested that the inhibitory effect of apigenin on phosphorylation of MAPKs, such as ERK (Mounho and Thrall, 1999; Kim et al., 2002; Yin et al., 2001) and p38 (Van Dross et al., 2003; Long et al., 2008). However, effects of apigenin on MAPKs are controversial (Frigo et al., 2002; Sarkar et al., 2004). For example, apigenin was reported to induce phosphorylation of both ERK and p38 kinase but there was no effect on the phosphorylation of JNK (Van Dross et al., 2003). Nevertheless, other articles showed that apigenin inhibited ERK1/2 activation (Kuo and Yang, 1995; Yin et al., 1999).
The expression level of MEKK1 is low in many cells. It is hard to observe alteration of signal transmission along a cascade in normal cells, especially the effect of chemicals on the pathway. To investigate the possible effects of apigenin on MAPKs in SM cells, we established a cell model of transient overexpression of MEKK1 by adenovirus infection. The virus-mediated overexpression of MEKK1 helped us to observe its activation of MAPKs and effects of apigenin on phosphorylation of MAPKs. In this study, we first activated/phosphorylated MAPKs by infecting SM cells with MEKK1-containing adenovirus. Then, the cells were exposed to apigenin and the activation levels of MAPKs were examined with Western blotting. The results show that MEKK1 overexpression in the SM cells activates ERK, JNK and p38 and that apigenin suppresses MEKK1's activation of the downstream molecules. This suggests that apigenin might block the signal transmission of MEKK1 to MAPKs and thereby inhibit cell movement.
It as widely accepted that cell movement is driven by actin polymerization. However, the detailed mechanism remains unclear. In the present study, we investigated the effect of apigenin on actin polymerization. Polymerized actin (F-actin) was visualized by Rhodamine-phalloidin. Results show that apigenin caused a decrease in polymerization filaments in most cells. For a smaller number of cells, apigenin did not affect actin polymerization. No significant difference was observed between untreated cells and MEKK1- overexpressing cell. The reason may be that actin polymerization is already activated in the culture environment, which is much different from in vivo. Cell culture in vitro is probably not a good model for research of actin polymerization.
In conclusion, by using MEKK1-expressing hBSM cells, we demonstrated that activation of MAPK pathway by MEKK1 triggered SM cell migration and that apigenin inhibited the movement. The mechanism might be that apigenin suppress signal transmission from MEKK1 to MAPKs.
Abbreviations: The abbreviations used are: ERK, extracellular signal regulated kinase; hBSM, human bladder smooth muscle; JNK, c-Jun amino-terminal kinase; MAP, mitogen-activated protein; MEK, MAP kinase/ERK kinase; MEKK1, MEK kinase 1; MLC 20, myosin light chain 20; PFU, plaque-forming unit.
Acknowledgements
This work is supported by grant sponsors: JSCAF Sci-Tech Innovation Talent Grant 2009C, Natural Science Foundation of China (30972484).
References
1. Ajay M, Gilani AU, Mustafa MR (2003). Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Science 5: 603-612. [ Links ]
2. Capasso R, Fiorino F, Ascione V, Frecentese F, Borrelli F (2006). Inhibition of rat vas deferens contractions by flavonoids in vitro. Journal of Pharmacy and Pharmacology 58: 381-384. [ Links ]
3. Chuang CM, Monie A, Wu A, Hung CF (2009). Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. Journal of Biomedical Science 16: 49. [ Links ]
4. Franzen CA, Amargo E, Todorovic V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC (2009). The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prevention Research (Phila) 2: 830-841. [ Links ]
5. Frigo DE, Duong BN, Melnik LI, Schief LS, Collins-Burow BM, Pace DK, McLachlan JA, Burow ME (2002). Flavonoid phytochemicals regulate activator protein 1 signal transduction pathways in endometrial and kidney stable cell lines. The Journal of Nutrition 132: 1848-1853. [ Links ]
6. Gharzouli K, Holzer P (2004). Inhibition of guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein. Pharmacology 70: 5-14. [ Links ]
7. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998). A simplified system for generating recombinant adenoviruses. Proceedings of the National Academy of Sciences of the United States 95: 2509-2514. [ Links ]
8. Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT (1999). A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. The Journal of Biological Chemistry 274: 24211-24219. [ Links ]
9. Henkels KM, Frondorf K, Gonzalez-Mejia ME, Doseff AL, Gomez-Cambronero J (2010). IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Letters 585: 159-166. [ Links ]
10. Hu XW, Meng D, Fang J (2008). Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis 29: 2369-2376. [ Links ]
12. Jeong GS, Lee SH, Jeong SN, Kim YC, Kim EC (2009). Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. International Immunopharmacology 9: 1374-1380. [ Links ]
13. Kim BR, Jeon YK, Nam MJ (2011). A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food and Chemical Toxicology 47: 1626-1632. [ Links ]
14. Kim TJ, Zhang YH, Kim Y, Lee CK, Lee MK, Hong JT, Yun YP (2002). Effects of apigenin on the serum- and platelet derived growth factor-BB-induced proliferation of rat aortic vascular smooth muscle cells. Planta Medica 68: 605-609. [ Links ]
15. Kim DI, Lee TK, Lim IS, Kim H, Lee YC, Kim CH (2005). Regulation of IGF-I production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: isolation of flavonoids of apigenin and luteolin as acting compounds. Toxicology and Applied Pharmacology 205: 213-224. [ Links ]
16. Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA (1997). Regulation of cell motility by mitogen-activated protein kinase. The Journal of Cell Biology 137: 481-492. [ Links ]
17. Kuo ML, Yang NC (1995). Reversion of v-H-ras-transformed NIH 3T3 cells by apigenin through inhibiting mitogen activated protein kinase and its downstream oncogenes. Biochemical and Biophysical Research Communications 212: 767-775. [ Links ]
18. Lamy S, Bedard V, Labbe D, Sartelet H, Barthomeuf C, Gingras D, Beliveau R (2008). The dietary flavones apigenin and luteolin impair smooth muscle cell migration and VEGF expression through inhibition of PDGFR-beta phosphorylation. Cancer Prevention Research (Phila Pa) 1: 452-459. [ Links ]
19. Lauffenburger DA, Horwitz AF (1996). Cell migration: a physically integrated molecular process. Cell 84(3): 359-369. [ Links ]
20. Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH (2008). Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicology and Applied Pharmacology 226: 178-191. [ Links ]
21. Li ZD, Hu XW, Wang YT, Fang J (2009). Apigenin inhibits proliferation of ovarian cancer A2780 cells through Id1. FEBS Lett 583: 1999-2003. [ Links ]
22. Long X, Fan M, Bigsby RM, Nephew KP (2008). Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Molecular Cancer Therapeutics 7: 2096-2108. [ Links ]
23. Morello S, Vellecco V, Alfieri A, Mascolo N, Cicala C (2006). Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Science 78: 825-830. [ Links ]
24. Mounho BJ, Thrall BD (1999). The extracellular signal-regulated kinase pathway contributes to mitogenic and anti-apoptotic effects of peroxisome proliferators in vitro. Toxicology and Applied Pharmacology 159: 125-133. [ Links ]
25. Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL (1999). Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. The Journal of Cell Biology 146: 149-164. [ Links ]
26. Noh HJ, Sung EG, Kim, JY, Lee TJ, Song IH (2010). Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by apigenin via the inhibition of p38 mitogen-activated protein kinase-dependent matrix metalloproteinase-9 expression. Oncology Reports 24: 277-283. [ Links ]
27. Rotondo A, Serio R, Mule F (2009). Gastric relaxation induced by apigenin and quercetin: analysis of the mechanism of action. Life Science 85: 85-90. [ Links ]
28. Rousseau S, Houle F, Landry J, Huot J (1997). p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15: 2169-2177. [ Links ]
29. Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JS, Case LM, Tessier-Lavigne M, Gaestel M, Cuenda A, Cohen P (2006). CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPKMAPKAP-K2-HSP27 pathway. Cell Signal 18: 1897-1905. [ Links ]
30. Sarkar FH, Li Y (2004). Cell signaling pathways altered by natural chemopreventive agents. Mutation Research 555: 53-64. [ Links ]
31. Van Dross R, Xue Y, Knudson A, Pelling JC (2003). The chemopreventive bioflavonoid apigenin modulates signal transduction pathways in keratinocyte and colon carcinoma cell lines. The Journal of Nutrition 133 (11 Suppl 1): 3800S-3804S. [ Links ]
32. Xia Y, Karin M (2004). The control of cell motility and epithelial morphogenesis by Jun kinases. Trends in Cell Biology 14: 94-101. [ Links ]
33. Yin F, Giuliano AE, Van Herle AJ (1999). Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO). Anticancer Research 19: 4297-4303. [ Links ]
34. Yin F, Giuliano AE, Law RE, Van Herle AJ (2001). Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Research 21: 413-420. [ Links ]
35. Yousufzai SY, Gao G, Abdel-Latif AA (2000). Mitogen-activated protein kinase inhibitors suppress prostaglandin F(2alpha)-induced myosin-light chain phosphorylation and contraction in iris sphincter smooth muscle. European Journal of Pharmacology 407: 17-26. [ Links ]
36. Zeigler ME, Chi Y, Schmidt T, Varani J (1999). Role of ERK and JNK pathways in regulating cell motility and matrix metalloproteinase 9 production in growth factor-stimulated human epidermal keratinocytes. Journal of Cellular Physiology 180: 271-284. [ Links ]
37. Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, Zheng Y, Xia Y (2005). MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Molecular and Cellular Biology 25: 60-65. [ Links ]
38. Zou Y, Chiou GC (2006). Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function. Journal of Ocular Pharmacology and Therapeutics 22: 425-430. [ Links ]
Received: August 8, 2011.
Accepted: November 15, 2011.














