Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell vol.36 no.1 Mendoza ene./abr. 2012
ORIGINAL ARTICLES
Optimization and comparison of two different 3D culture methods to prepare cell aggregates as a bioink for organ printing
Rana Imani1, Shahriar Hojjati Emami1, Hossein Fakhrzadeh2, Nafiseh Baheiraei1 and Ali M Sharifi* 2,3,4
1Department of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
2Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
3Razi Institute for Drug research, Department of Pharmacology , School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
4Department of Tissue Engineering and Cell therapy, School of advanced sciences in Medicine, Tehran University of Medical Sciences. Tehran, Iran.
*Address correspondence to:
Ali Mohammad Sharifi.
E-mail: sharifal@yahoo.com ; amsharifi@TUMS.ac.ir
ABSTRACT: The ultimate goal of tissue engineering is to design and fabricate functional human tissues that are similar to natural cells and are capable of regeneration. Preparation of cell aggregates is one of the important steps in 3D tissue engineering technology, particularly in organ printing. Two simple methods, hanging drop (HD) and conical tube (CT) were utilized to prepare cell aggregates. The size and viability of the aggregates obtained at different initial cell densities and pre-culture duration were compared. The proliferative ability of the cell aggregates and their ability to spread in culture plates were also investigated. In both methods, the optimum average size of the aggregates was less than 500µm. CT aggregates were smaller than HD aggregates. 5,000 cells per drop HD aggregates showed a marked ability to attach and spread on the culture surface. The proliferative ability reduced when the initial cell density was increased. Comparing these methods, we found that the HD method having better size controlling ability as well as enhanced ability to maintain higher rates of viability, spreading, and proliferation. In conclusion, smaller HD aggregates might be a suitable choice as building blocks for making bioink particles in bioprinting technique.
Key words: Tissue engineering; Hanging drop; Conical tube; Bioprinting
Introduction
Tissue engineering is the process of designing and constructing artificial tissues and organ replacements. The ultimate goal of tissue engineering is to design and fabricate functional human tissues and organs that are similar to natural cells and organs, thereby allowing regeneration, repair, and replacement of damaged, injured, or lost organs. To date, tissue engineering studies have mainly focused on using biodegradable scaffolds as a spatial support for the assembly of isolated cells into tissues. Insights from developmental biology suggest that instead of attempting precise engineering of the final tissue structure, tissue engineers should try to produce scaffolds or create microenvironments that mimic those of natural tissues and promote complex cell-cell and cell-matrix interactions (Mironov et al., 2009). Thus, a biomimetic approach to tissue engineering, or simulation of some aspects of normal tissue development and remodeling, could be a key element to achieve success in this field (Ingber et al., 2006).
Cellular self-assembly, the most fundamental mechanism in the origin of life and the evolution of complex biological organs, exists at all levels in living systems (Mironov et al., 2003). Using this mechanism, cells attach to each other and form three-dimensional (3D) spheroids. In comparison to cells in monolayer cultures, cells that self-assemble into spheroids achieve elevated gene expression, while maintaining the native cell phenotype. These cells show natural cell-cell interactions and mimic in vivo differentiation patterns and spatial cell-cell and cell-extracellular matrix (ECM) interactions (Napolitano et al., 2007). Self-assembling cell aggregates may provide a better starting point and result in faster organ formation.
In addition to self-assembling, tissue spreading, an important process in organ development and wound healing, is critically important in the emerging field of tissue engineering and for the future use of biomaterial scaffolds for organ regeneration (Ryan et al., 2001). Studies on the cell-cell and cell-surface adhesion could contribute to the rational design of scaffold materials. Regulation of tissue-spreading movements by differential cell cohesion and adhesion has been demonstrated in embryonic morphogenesis (Ryan et al. 2001). Thus, controlled tissue spreading is essential to achieve fusion and structure formation.
Recent studies have recommended the use of cell aggregates instead of single or monolayer cells as building blocks in tissue engineering (Xu et al., 2010; Rosines et al., 2010). The spherical cell aggregates with many thousands of cells has also been proposed as an alternative to individual cells (Jakab et al., 2004). Current advanced studies, e.g. cell and organ printing, have focused on scaffold-free tissue engineering using self-assembled micro tissues (Kelm et al., 2010).
Organ printing, defined as layer-by-layer bio-manufacturing, is an emerging biomimetic technology that has the potential to overcome the limitations imposed by traditional solid scaffold-based tissue engineering. Spherical cell aggregates can be considered as bioink particles for the organ printing approach. This technology requires spherical and uniform droplets, which can be easily stored and accurately extruded from printer cartridges (Niklason and Langer, 2001). Therefore, preparation of cell aggregates is an essential step in 3D tissue engineering. Despite the importance of aggregate preparation, there is no evidence indicating the optimum conditions for the production of suitable aggregates.
Three dimensional culturing of cells produces distinct cell morphology and signaling events, in contrast to those observed in a rigid 2-dimensional (2D) culture system. Although cellular spheroid generation has been utilized in many applications dealing with pharmaco-logical or cancer-related issues (Kelm et al., 2003), more recently, 3D culture systems have been widely used in biomedical research (Winters et al., 2006). Different methods for the preparation of tissue spheroids and cell aggregates have been developed (Lin and Chang, 2008). In order to identify the ideal method of aggregate preparation for large-scale tissue engineering and for organ printing, it is essential to formulate appropriate criteria or well-defined specifications.
Therefore, the objective of this study was to prepare, characterize, and optimize cell aggregates as building blocks of tissue constructs for application in current cell-based tissue engineering approaches such as organ printing. Two simple methods-hanging drop (HD) and conical tube (CT)-were examined and compared in this regard. The shape, size, and cell viability of the aggregates obtained using different initial cell density values with both methods were compared. We also investigated the proliferative and cell-spreading abilities of the aggregates.
Material and Methods
Cell culture
Chinese Hamster Ovary (CHO) cells were obtained from Pasteur (Tehran, Iran) and maintained in RPMI1640 (Gibco, Invitrogen) medium supplemented with 10% (v/v) fetal bovine serum (Gibco, Invitrogen) and 1% penicillin-streptomycin (100 U/ml penicillin and 100µg/ml streptomycin; Gibco, Invitrogen) in a 90% humidified incubator with 5% CO2 in air at 37°C. The culture medium was changed every 48 h, and the cells were subcultured every 2-3 days. Cells were harvested in the sub-confluent stage, and a cell suspension was prepared for subsequent aggregate preparation.
Preparing spherical aggregates
Two different methods, HD and CT, with various initial cell densities were used for the preparation of cell aggregates. For the HD culture technique, 20-µL drops of each prepared cell suspension containing approximately 5,000, 10,000, 25,000, or 50,000 cells per drop were pipetted onto the inner side of the lid of a 15-cm diameter tissue culture Petri dish. After distribution of the drops, the lid was gently inverted and placed over a Petri dish containing 4 mL of phosphate-buffered saline (PBS) to humidify the culture chamber. Hanging drops were incubated under tissue culture conditions, allowing the cells to coalesce at the base of the droplets and to form aggregates (Fig. 1b).

FIGURE 1. 3D aggregate culture methods, (a) Conical tube (b) Hanging Drop.
In the CT technique, 200µL of each cell suspension, containing 5,000, 10,000, 25,000, or 50,000 cells per drop, was placed in 200-µL microtubes and centrifuged at 2,000 rpm for 5 min, to accelerate the aggregation. The resulting cell pellet was left undisturbed at the bottom of the tube. The microtube cap was left loose to allow supply of oxygen and incubated under tissue culture conditions (Fig. 1a).
Analysis of the aggregate size by microscopy and imaging
Cells were left at the preparation stage for a maximum of 5 days to analyze the effect of initial cell density (cells per well and cells per drop) and pre-culture time on the size of the aggregates. The time course of aggregate preparation using the HD and CT methods were examined. The aggregates were photographed by an inverted light microscope with a camera (Olympus, Japan). The images were analyzed using an image analyzing software (Motic Image Proplus) to determine the changes in the size (radius) of the aggregates at various time intervals and initial cell densities.
Analyzing cell viability of the aggregates
CT and HD aggregates were removed from the culture environment and enzymatically dispersed with 0.15% trypsin to obtain single cells. Mild mechanical force using a pipette was also applied to facilitate dispersion. Cell viability of the aggregates was determined by the Trypan blue exclusion test (Cavallari et al., 2007).
Aggregate cell spreading and proliferation test
To examine the ability of the tissue to spread and interact with an adhesive substrate (e.g. scaffolds), the radius of the area covered by the cells on the surface of the tissue culture plate was measured by microscopic imaging. The ratio of the radius of the spread of cells to that of the initial aggregate was calculated as an expansion parameter (Re/Ri). For this purpose, on the third day of pre-culture, the cells were transferred onto the surface of a tissue culture plate with culture medium and were further incubated for 3 days.
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumboromide) assay was used to estimate the number of cells and their proliferation with respect to initial cell density. A standard calibration curve was prepared to estimate the exact number of viable cells per well. Then, pre-culture aggregates (the more viable ones) were transferred to a 96-well plate on the third day. After 3 days of further incubation, 20µl of MTT solution was added to each well, and the cells were incubated for 4 hours. Then, 200µl dimethyl sulfoxide (DMSO) was added to the wells to solubilize the precipitated dye. Absorbance was measured by using an ELISA reader set at 570 nm. In the last step, the number of viable cells in each well was estimated by comparing the absorbance to the values of the standard curve. The values obtained represent the cell proliferation during 6 days (3-day pre-culture followed by 3 days of culture).
Statistical analysis
All the results were expressed as mean ± standard deviation (SD). Comparisons between the 2 methods (HD and CT) for different initial cell densities were performed by analysis of variance (ANOVA) and Tukey's test, and those for proliferation and cell spreading data were performed by independent sample t-test. Statistical analyses were performed using SPSS software 16.0, statistical significance was set at P < 0.05.
Results
Aggregate formation
Aggregate formation was first observed on the second and third day of pre-culture, respectively, for HD and CT. In CT, 2 or more small aggregates appeared in the intermediate stage (second day). Finally, a single body was formed at the bottom of the conical tube in most cases. Data obtained by investigation of aggregate size over a period of 5 days are shown in Fig. 2. With a gradual decrease in the radius of the HD aggregates, shape of the surrounding aggregates became rounder and smoother. After 48 h of pre-culture, smooth-shaped ag-gregates were formed in most drops (Fig. 3a). The CT aggregates generally had an irregular shape (Fig. 3b).
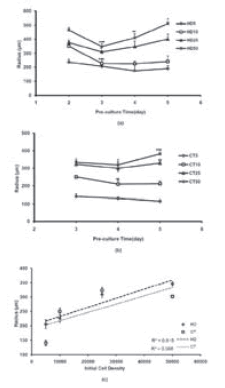
FIGURE 2. Aggregate size analysis, (a) and (b): Size changing of aggregate during pre-culture period. (c): Size changing versus of initial cell density ( data indicate aggregates radius in day 3).Values are average ± standard deviation (n=4). For investigating the trend of size changing each day compares to previous day. (*, **, ***, represent P <0.05, P <0.01 and P <0.001 repectively).
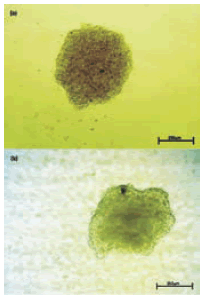
FIGURE 3. Formed aggregate during pre-culture time. Images are at identical magnification. (a): CT5-day 4, (b): HD5-day 3(Scale bar: 250µm).
Size analysis
In both the methods, size analysis data for most of the aggregates, showed a biphasic pattern of initial reduction, followed by an enhancement in size (Fig. 2). The observed minimum aggregate size for each method was different. For HD25 and HD50, the minimum aggregate size was observed on the third day, after which a significant increasing in size was observed (Fig. 2a). For HD5 and HD10, the size decreased till the fifth day of pre-culture, after which there was no significant increase in the size. The minimum aggregate size for CT5, CT10, and CT25 was observed on the fourth day. The trend of size change in these 3 groups was nearly monophasic, and the size of the aggregates continually decreased till the fifth day. However, aggregates in the CT50 group showed a significant increase in size on the fifth day (P < 0.001). Although the minimum aggregate size during pre-culture was lower than 500µm in both the methods, cell aggregates formed by the 2 smaller cell density aggregates, 5,000 and 10,000, had a more ideal size (<250µm).
Cell viability
Cell viability decreased during the incubation period. In all the aggregates, the viability reduction rate was slow initially and accelerated thereafter. In HD aggregates, (Fig. 4a), the viability reduction rate significantly accelerated on the third day (HD10, HD25, and HD50) and the fourth day (HD5); further, for CT aggregates, this trend showed a considerable drop on the third day (CT5 and CT10) and the fifth day (CT25 and CT50) (Fig. 4b). On comparing the cells of the CT and HD aggregates, we found that the former were less viable at the same initial cell density and pre-culture time. This difference was significant, particularly at higher cell densities (Fig. 4c).

FIGURE 4. Percent of aggregates viability during pre-culture. (a):HD, (b):CT (for each sample, percent of viability in each day compares to previous day). (c): comparison between HD and CT at given initial cell desity, 25000 (there are significant differences between CT and HD in all days except day5). Values are average ± standard deviation (n=4). (*, **, ***, represent P <0.05, P <0.01 and P <0.001 repectively).
Cell spreading and proliferation
We have developed a straightforward and versatile assay to quantify Cell spreading and proliferation and have used tissue spreading to investigate the other factors controlling cell cohesivity. Cell spreading from an attached aggregate could be observed on the third day of the initial culture. However, cell spreading and migration from explant aggregates towards surrounding areas in culture dishes were time-dependent. (Fig. 5) A wider area of spreading correlated with a lower density in the initial aggregates. The expansion parameter for CT and HD aggregates are indicated in Table 1. On the basis of the results, the expansion parameter was found to be more significant for HD5 and CT5 than for the others (Fig. 6). On comparing the 2 methods at similar periods of time, cells of the HD aggregates showed a higher ability to attach and spread on a culture dish. In CT25, CT50, and HD50, no detectable expansion was observed even after the fourth day of culture. The spread-ing rate was directly associated with the initial cell density; the higher the cell density, lower the extent of the spread.

FIGURE 5. Cell spreading over culture plate for HD5 after 1 day (∞100). (Scale bar: 250µm).
TABLE 1. Comparing extension parameter for different samples in different culture days, (a) for HD, day4, there are significant differences between all groups, (b) for CT, day4, there are significant differences between CT5 and other groups. *** represent P<0.001. Values are average ± standard deviation (n=3).
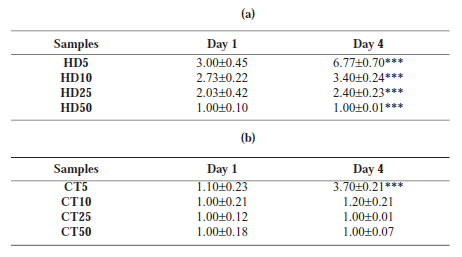
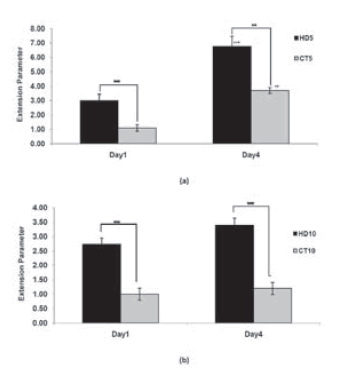
FIGURE 6. Comparing expansion parameter between HD and CT samples after1 and 4 days culture. Values are average ± standard deviation (n=3). ** and *** represent P <0.01 and P <0.001 respectively .+ and ++ compare extension parameter between two different days and represent P <0.01 and P <0.001 respectively.
We examined the proliferative abilities of the cells of the more viable aggregates (HD5 and HD10, CT5 and CT10). The proliferation rate of the spreading cells decreased when the initial cell density was increased. CT5 and HD5 produced 9- and 12.44-fold increase in cell number, respectively, in 72 h; for CT50 and HD50, the increase in cell number was less than 1 fold (Fig. 7).

FIGURE 7. Effect of initial cell density on Proliferation factor for CT and HD aggregates characterized. Values are average ± standard deviation (n=3). There is significant difference between CT and HD aggregates, particularly in lower cell density (** and *** represent P <0.01 and P <0.001 respectively).
Discussion
An understanding of biomimicking and developmental mechanisms involved in embryonic organogenesis can be very beneficial for tissue engineering strategies. Using the fundamental principles derived from these studies, tissue engineers will be able to design therapeutic approaches that are based on the way these structures are built in nature. As implied in other studies, micro tissues, and more specifically tissue aggregates, can also be considered as "living material" that can mimic the architectural and functional characteristics of native tissues. Compared to conventional mono-layer cultures, multicellular spheroids are more representative of real tissues in terms of structural and functional properties. On the basis of this point of view, novel tissue engineering technologies like organ printing have been tried to utilize cell aggregates as building blocks of tissue constructs. Therefore, one of the essential stages in organ printing is the preparation of cell aggregates, which can serve as bioink particles.
Different studies have proposed the use of cell aggregates, along with ECM proteins, as building blocks in organ-building strategies based on inkjet printer technology (Jakab et al., 2004; Boland et al., 2003; Norotte et al., 2009) or in conventional solid scaffold based tissue-engineering processes like utilizing aggregates of fibroblasts to create tissue-engineered skin (Dai and Saltzman, 1996).
Preformed aggregates that undergo fusion may be more advantageous than single cells for several reasons. Aggregates may show better viability after undergoing handling procedures; they already have a high cell density similar to that of native tissue, and they create an immediate 3D structure upon assembly, thereby, reducing the time necessary to construct a structure (Cavallari et al., 2007).
Because of the remarkable progress in research on multicell systems and the increasing interaction between researchers working in different fields of biomedical science, like tissue engineering, and using similar 3D culture technique, the potential of 3D cell cultures is currently being exploited in many areas of biomedical research. Although different 3D culture methods have already been investigated for preparing cell aggregates and micro tissues, conventional laboratory methods like hanging drop or pellet culture conical tube are also used.
In this study, in order to prepare aggregates from CHO, a widely used and well characterized cell line in bioprinting researches (Jakab et al. 2006, 2007; Xu et al., 2005), 2 methods, the HD and the CT, were employed.
HD cultures are being widely used to form embryonic bodies. This method was already applied to a wide range of cellular systems and tissue engineering. In terms of cancer biology, hanging drop method was applied for the formation of multicellular spheroids using a wide range of cell lines with structural analyses for the formation of tissue-like structures (Kelm et al., 2003). Concrete applications of the hanging-drop method to generate scaffold-free three-dimensional (3D) structures from in vitro expanded chondrocytes in the tissue-engineering field have also been described (Martinez et al., 2008). Moreover, the method has been applied to kidney-like tissues for in vitro tissue engineering (Rosines et al., 2010). The cells accumulate at the bottom of the drop due to gravity and join together to form an aggregate (Fig. 1a) (Marga et al., 2007). The rounded bottom of a hanging drop can provide a good environment for the formation of an aggregate. The CT method is another simple method used to create aggregates, which can be formed by means of the rounded bottom of a polypropylene conical tube (Marga et al., 2007). The use of polypropylene is critical in this technique; it provides a nonadhering surface for the pellet, but retains the cell-cell interactions to yield free-floating cell aggregates (Fig. 1b). In comparison with other methods, these methods afford greater controllability of the cell number in the aggregates, which can be controlled by altering the cell number in the initial cell suspension (Cavallari et al., 2007).
On the basis of the data obtained, the process of aggregate formation can be explained as follows. This is inherently a 3-step process consisting of an integrin/ ECM fibermediated phase, a delay phase in which cadherin expression is upregulated, and an E-cadherin-mediated compaction phase. Any method that concentrates suspended cells to yield a high-density sediment can potentially facilitate aggregate formation, particularly in poorly aggregating cells (Lin and Chang, 2008). In comparison to the HD method, in which the cells sedimented freely by the force of gravity, centrifugation of cells immediately forces them to form aggregates. However, as some studies have pointed out (Wel-ter et al., 2007), exposure to elevated g forces is not an inert process; it has the potential to stimulate or inhibit cellular events in ways, which may interfere with the processes being studied. In this study, the aggregates were formed and observed on the second and third day for HD and CT, respectively. The optimum time for aggregate formation and utilization was the third day for HD25 and HD50; fourth day for HD5 and HD10; and fourth day for most of the CT aggregates.
Along with formation, the size is a critical aggregate characteristic. It strongly affects cell viability, particularly by affecting the oxygen and nutrient availability to the central portion of the aggregate. Moreover, controlling the aggregate size by using automatic cell dispensers or cell printers used for bioprinting is necessary for their effective deposition. On the basis of the size-analysis data, a biphasic pattern of initial reduction and consequent enhancement were shown in this study (Fig. 2). Decrease in size is normally the result of higher cell-cell cohesion, which yields more compact aggregates. Increase in size could be a consequence of loss of cell cohesion caused by death in internal parts of the aggregate, which has been demonstrated in the cell viability data (Fig. 4). The change in size caused by changes in the initial cell density seems directly proportional in the case of HD aggregates; therefore, size controllability with this method is more achievable than with the CT method (Fig. 2c).
Our results indicated that the cells obtained by using the CT method were less viable than those obtained with the HD method. This difference could be explained by the access of the cells to oxygen and nutrients. HD aggregates formed at the interface between the drops the air could have access to more oxygen, whereas CT aggregates formed at the bottom of the microtube have less access to sufficient oxygen.
From tissue engineering point of view, cells in aggregates placed on scaffolds show 2 types of interactions: cell-cell cohesion and cell-surface adhesion. Some studies have indicated that when the aggregate is too cohesive, cells cannot migrate, whereas if the aggregate is not sufficiently cohesive, the cells will disperse into the 3D substrate (Ryan et al., 2001). On comparing the data obtained for the expansion parameter, we found that at the same initial cell density, the spreading of cells from HD aggregates was markedly faster than that of the cells from CT aggregates. Therefore, we concluded that the CT method yields more coherent aggregates, while HD aggregates significantly interact with the substrate. In addition, aggregates formed with a higher number of initial cells showed a lower tendency to adhere to the surface and spread. Contact between the aggregate and the substrate is essential for tissue spreading, and therefore, the reactive surface area of the aggregate is critical. Mathematical modeling suggests that the surface area is inversely related to microtissue stability; the greater the surface area, the higher the free energy of the system. Therefore, an aggregate with a higher surface area might be expected to react more quickly to reduce its overall free energy (Rago et al., 2009). We speculated that smaller aggregates with fewer numbers of cells attached faster to the surface. Another factor that may increase the spreading rate of small aggregates is the less distance that the cells would have to cover to move towards the perimeter, which would allow them to reach the surface faster and spread. This study shows that the aggregate preparation method and initial cell density can influence cell-cell cohesivity and consequently, tissue spreading.
Cell proliferation, as an essential phenomenon in tissue construction, was examined using the MTT assay. The proliferation rate of cells decreased with an increase in the initial cell density of the aggregates. This resulted in a 9- and 12-fold increase in cell number in smaller CT and HD aggregates, respectively (Fig. 7). The MTT data corresponded to the tissue-spreading data, thereby showing that the proliferation rate increased as a consequence of better cell spreading in less cohesive aggregate. The reason for the less than 1-fold increase in cell number for HD50 and CT50, shown in Table 1, could be cell death during pre-culture time.
Considering all the results obtained, we concluded that compared to CT, the HD method is more efficient in generating spherical aggregates from single-cell suspensions within a short period of time. In particular, this method particularly provides more homogenous aggregates, better size controlling ability, and a better capability to maintain higher cell viability.
Finally, the smaller HD aggregates could maintain their intrinsic ability of attachment, migration, spreading, and proliferation better than the CT aggregates and therefore, these HD aggregates could be a suitable choice for bio-printing.
Acknowledgment
This study was funded by a grant provided from Endocrinology and Metabolism Research Center, Tehran University of Medical Sciences.
References
1. Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR (2003). Cell and organ printing 2: fusion of cell aggregates in three-dimentional gels. The Anatomical Record Part A 272A:497-502. [ Links ]
2. Cavallari G, Zuellig RA, Lehmann R, Weber M, Moritz W (2007). Rat pancreatic islet size standardization by the hanging drop technique. Transplantation Proceedings 39: 2018-2020. [ Links ]
3. Dai W, Saltzman WM (1996). Fibroblast aggregation by suspension with conjugates of poly(ethylene glycol) and RGD. Biotechnology and Bioengineering 50: 349-356. [ Links ]
4. Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, Yannas I, Kaplan D, Vunjak-Novakovic G (2006). Tissue engineering and developmental biology: going biomimetic. Tissue Engineering 12: 3265-3283. [ Links ]
5. Jakab K, Neagu A, Mironov V, Forgacs G (2004). Organ printing: fiction or science. Biorheology 41: 371-375. [ Links ]
6. Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G (2006). Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proceedings of the NationalAcademy of Science (U S A) 101: 2865-2869. [ Links ]
7. Jakab K, Norotte C, Damon B, Maraga F, Neagu A, Besch-Williford CL, Kachurin A, Church KH, Park H, Mironov V, Markwald R, Vunjak-Novakovic G, Forgacs G (2007). Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Engineering Part A 14:413-421. [ Links ]
8. Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zünd G, Hoerstrup SP (2010). A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. Journal of Biotechnolgy 148: 46-55. [ Links ]
9. Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK (2003). Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and Bioengineering 83: 173-180. [ Links ]
10. Lin RZ, Chang HY (2008). Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Bio-technology Journal 3:1172-84. [ Links ]
11. Marga F, Neagu A, Kosztin I, Forgacs G (2007). Developmental biology and tissue engineering, Birth Defects Research (Part C) 81: 320-328. [ Links ]
12. Martinez I, Elvenes J, Olsen R, Bertheussen K, Johansen O (2008). Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplantation 17: 987-996. [ Links ]
13. Mironov V, Markwald R, Forgacs G (2003). Organ printing: self assembling cell aggregate as cellular aggregate bioink. Science & Medicine 9: 69-71. [ Links ]
14. Mironov V, Visconti R P, Kasyanov V, Forgacs G, Drake CJ, Markwald RR (2009). Organ printing: Tissue spheroids as building blocks. Biomaterials 30: 2164-2174. [ Links ]
15. Napolitano A P, Chai P, Dean DM, Morgan JR (2007). Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Engineering 13: 2087-2094. [ Links ]
16. Niklason LE, Langer R (2001). Prospects for organ and tissue replacement. Journal of the American medical association 285: 573-576. [ Links ]
17. Norotte C, Marga FS, Niklason LE, Forgacs G (2009). Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30: 5910-5917. [ Links ]
18. Rago A P, Dean DM, Morgan JR (2009). Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Biotechnology and Bioengineering 102: 1231-1241. [ Links ]
19. Rosines E, Johkura K, Zhang X, Schmidt HJ, Decambre M, Bush KT, Nigam SK (2010). Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney. Tissue Engineering Part A 16: 2441-2455. [ Links ]
20. Ryan PL, Foty RA, Kohn J, Steinberg MS (2001). Tissue spreading on implantable substrates is a competitive outcome of cell-cell vs. cell-substratum adhesivity. Proceedings of the NationalAcademy of Science (U S A) 98: 4323-4327. [ Links ]
21. Welter J F, Solchaga LA, Penick KJ (2007). Simplification of aggregate culture of human mesenchymal stem cells as a chon-drogenic screening assay. Biotechniques 42: 734-737. [ Links ]
22. Winters BS, Raj BK, Robinson EE, Foty RA, Corbett SA (2006). Three-dimensional culture regulates Raf-1 expression to modulate fibronectin matrix assembly. Molecular Biology of the Cell 17: 3386-3396. [ Links ]
23. Xu F, Moon SJ, Emre AE, Turali ES, Song YS, Hacking SA, Nagatomi J, Demirci U (2010). A droplet-based building block approach for bladder smooth muscle cell (SMC) proliferation. Biofabrication 2: 014105. [ Links ]
24. Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005). Inkjet printing of viable mammalian cells. Biomaterials 26:93-99 [ Links ]
Received: October 13, 2011.
Revised version received: March 21, 2012.
Acepted: March 15, 2012.














