Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Biocell
versão impressa ISSN 0327-9545
Biocell vol.36 no.2 Mendoza ago. 2012
ORIGINAL ARTICLES
A study of chlorophyll-like and phycobilin pigments in the C endosymbiont of the apple- snail Pomacea canaliculata
Israel A. Vega*1,2, Federico A. Dellagnola1, Jorge A. Hurst3, Martín S. Godoy1 and Alfredo Castro-Vazquez1,2
1 Laboratorio de Fisiología (IHEM-CONICET), and Departamento de Morfología y Fisiología (Facultad de Ciencias Médicas, Universidad Nacional de Cuyo), Casilla de Correo 33, 5500 Mendoza, Argentina.
2 Instituto de Ciencias Básicas - Universidad Nacional de Cuyo. Padre Jorge Contreras 1300. Parque Gral. San Martín, 5500 Mendoza.
3 Departamento de Química Biológica. Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires. Present address: Instituto de Botánica Darwinion. Labardén 200, B1642HYD Buenos Aires.
*Address correspondence to:
Israel A. Vega.
Area Fisiología. IHEM-CONICET. Casilla de Correo 33, (5500) Mendoza, Argentina. Phone/fax: +54 261 449 4117 E-mail: israel.vega7@gmail.com
Abstract: Pigments present in the brown-greenish C morph of an intracellular endosymbiont of Pomacea canaliculata were investigated. Acetone extracts of the endosymbiotic corpuscles showed an absorption spectrum similar to that of chlorophylls. Three fractions obtained from silica gel column chromatography of the acetone extracts (CI, CII and CIII), were studied by positive ion fast atom bombardment-mass spectrometry (FAB-MS) and hydrogen-nuclear magnetic resonance (H-NMR). Results indicated the presence of (1) a sterol in the yellow colored CI fraction; (2) a mixture of pheophorbides a and b in the major green fraction, CII; and (3) a modified pheophorbide a in the smaller green fraction, CIII. Aqueous extracts of the C endosymbiont did not show evidence of the occurrence of C-phycocyanin, allophycocyanin or phycoerithrin (light absorption, fluorescence emission, and electrophoresis of the protein moieties) while cyanobacterial cells (Nostoc sp.) showed evidence of C-phycocyanin and allophycocyanin. The possible phylogenetic and functional significance of the pigments present in the C endosymbiont is discussed.
Key words: Symbiosis; Cyanobacteria; Chloroplast; Photosynthetic pigments; Phylogeny
Introduction
Pomacea canaliculata (Architaenioglossa, Ampullariidae) is a freshwater snail original from the Plata basin (Hayes et al., 2008) but that it has spread to South East Asia, China, Japan and Hawaii (Rawlings et al., 2007) where it has become a pest of rice and other crops (Halwart, 1994; Cowie, 2002).
Brown-greenish corpuscles of an obligate endosymbiont are contained within specific cells of the midgut gland of this snail, where they appear to reproduce, and are later released in the gut lumen and are expelled in the feces (Vega et al., 2006).
A preceding investigation has suggested the occurrence of chlorophyll-like pigments in both the C and K morphs of that endosymbiont (Castro-Vazquez et al., 2002), and this work was aimed to follow up that study in the C morph, which seems to be the vegetative form (Castro-Vazquez et al., 2002; Koch et al., 2006). The identity of the pigments contained within the symbiotic corpuscles may shed light on the phylogenetic position of the endosymbiont, and of the fuctional meaning of its consortium with P. canaliculata. For such purpose we have used light absorption, fluorescence emission, positive ion fast atom bombardment-mass spectrometry (FAB-MS), hydrogen-nuclear magnetic resonance (H-NMR) and protein electrophoresis (SDS-PAGE) of extracts of the isolated C corpuscles (Vega et al., 2005).
Materials and Methods
Animals
Adult female snails from a cultured strain of P. canaliculata were used. The origin of animals and the culturing conditions were those described elsewhere (Vega et al., 2005).
Materials used
All solvents used were HPLC grade. Acetone was distilled on sodium carbonate before use. Thin layer chromatography plates were from Merck (Z292990), Tris (ICN; 819620), NaCl (Merck, 1.06404.1000.1026) and EDTA (ICN 800682) were used to isolate the C endosymbiont.
Isolation of C corpuscles from midgut gland tissue
First the animals were immersed in an ice bath during 10 min. The midgut gland was then dissected and the C corpuscles were isolated according to the method previously described (Vega et al., 2005). Purity of fractions was microscopically controlled and only those C fractions containing less than 5% of K corpuscles (the putative kystic form of the endosymbiont) were weighted and freeze-dried in plastic tubes and stored at -80°C until their use for pigment extraction.
Acetone extraction of C corpuscles
C fractions of the midgut glands of 50 animals were weighted and pooled, resuspended in 9 mL of acetone per gram of tissue and then homogenized (Ultraturrax® homogenizer) for 1 minute (this and the following procedures were performed at 4ºC in darkness). The homogenate was centrifuged at 750 g for 5 minutes and the supernatant was kept in the cold. Then, the precipitate obtained was again resuspended in an acetone-water solution (1:9; v/v), homogenized and centrifuged, and the second supernatant was collected and mixed with the first supernatant. A sample of the brown-greenish supernatants was used for light absorption spectrum analysis while the remaining extract was evaporated under a nitrogen flow and stored at -80ºC until further processing.
Pigments in a sample of the acetone extract were first separated by thin layer chromatography and the Rf values of the observed bands were determined. Three bands were thus identified as CI (Rf = 0.97), CII (Rf = 0.84) and CIII (Rf = 0.51). The CI band was yellow while CII and CIII were green (CII was larger than CIII).
The corresponding fractions in the remaining extracts were then purified by silica gel column chromatography, using nitrogen to push the solvents through the column. The solvent used was chloroform, and its polarity was increased by the addition of methanol (1-3%). The fractions thus obtained were studied by (1) positive ion fast atom bombardment mass spectrometry (FAB-MS) and (2) hydrogen-nuclear magnetic resonance (H-NMR).
Aqueous extraction of C corpuscles
Phycobiliproteins were extracted according to the method of Bennett and Bogorad (1973) with minor modifications. All procedures were performed in darkness at 4ºC. Midgut gland fractions containing C corpuscles were obtained from 4 snails and were controlled for purity as described above. The fraction from each snail was dispersed in phosphate-buffered saline solution (10 mM phosphate buffer, 150 mM NaCl, pH=7; 1 mL per gram of fraction). Then the samples were sonicated with the microtip of a Systems model W185D sonifier (Heat Systems-Ultrasonics, Inc., Plainview, NY) operated at power setting 5; each sample was exposed to 16 cycles of 10 seconds duration, alternating with 1 min periods for cooling at 4ºC. Sonicates were then centrifuged at 100,000 g for 45 minutes; the precipitate was discarded and the supernatant was kept at 4ºC. Cells of an axenic Nostoc sp. culture (Cyanobacteria, Nostocales) were similarly processed and used as positive controls.
The supernatants from both C corpuscles and Nostoc sp. cells were then used to determine phycobiliproteins through (1) light absorption spectrum (400-700 nm), (2) fluorescence emission spectrum after excitation at 615 nm (for C-phycocyanin), 652 nm (for allophycocyanin) and 575 nm for phycoerithrin, and (3) electrophoresis (SDS-PAGE) of the protein moieties.
Soluble proteins in the supernatants from both C endosymbiont and Nostoc sp. were determined by the Lowry et al. (1951) method and were separated by SDS-PAGE (10% SDS, 15% polyacrylamide) according to Sambrook et al. (1989). Each sample was diluted at 14 µg of soluble proteins in sample buffer (50 mM Tris-HCl, 2% SDS, 0.1% Bromophenol Blue and 10% glycerol, 5% β-mercaptoethanol, pH 6.8). Each gel was run at 30 mAmp using a Mini Protean II gel system (BIORAD) and then stained with a 0.75% solution of Coomassie Brilliant Blue R-250 in methanol-acetic acid (90:10, v/v).
Results
Ligth absorption spectra of acetone extracts of C corpuscles and Nostoc sp.
The absorption spectra (between 400 and 700 nm) of the acetone extracts of C endosymbiont (continuous line) and Nostoc sp. (dashed line) are shown in Fig.1. The values are expressed as percent of the maximum absorption peak. The light absorption spectrum of C corpuscles was characterized by a maximum peak at 420 nm followed by two shoulders at 440 and 470 nm. Also, smaller peaks were observed at 540, 605, and 670 nm. Nostoc sp. cells showed a similar light absorption pattern (Fig. 1; dashed line) though the maximum peak shifted from 420 to 430 nm. Also, Nostoc sp. showed the second largest peak at 660 nm (i.e., 10 nm to the left of the corresponding peak in the C corpuscles spectrum).

Figure 1. Ligth absorption spectrum of an acetone extract of C corpuscles isolated from the midgut gland of P. canaliculata (continuous line) and of an axenic culture of Nostoc sp. (dashed line). Results were expressed as percent of the highest absorbance value in each case.
FAB-MS and H-NMR studies of the column chromatography fractions
The FAB-MS pattern of the CI fraction shared 10 of 15 mass fragments with those of cholesterol (Fig. 2).
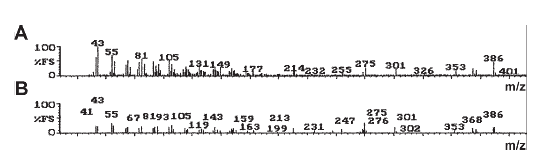
Figure 2. FAB-MS of the CI fraction obtained from the acetone extract of C corpuscles (A) and its comparison with the fragmentation pattern of cholesterol (B).
The CII fraction FAB-MS and H-NMR spectra are shown in Figs. 3 and 4, respectively. The FAB-MS spectrum (Fig. 3) showed an intact molecular mass at m/z 603 with a relative intensity of 43%. Several fragmentation peaks were observed at m/z 577 (28%), 549 (8%), and 459 (4%) (relative intensities in parenthesis), which suggest the loss of (1) an ethyl group in C8 (m/z 577), (2) a carboxyl-methyl group in C133 (m/z 549), and (3) a propionic chain in C17 position (m/z 459).

Figure 3. FAB-MS of the CII fraction obtained from silica gel column chromatography of the acetone extract of C corpuscles.

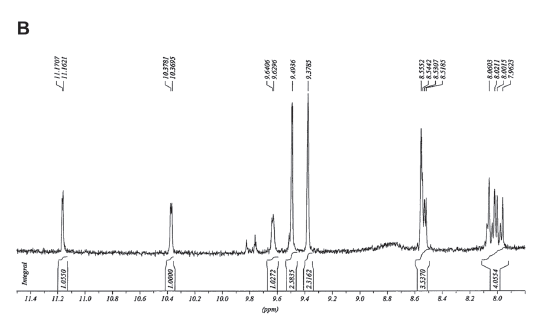

Figure 4. A. H-NMR spectrum of the CII fraction obtained from silica gel column chromatography of an acetone extract of C corpuscles. Several regions of the spectrum were amplified in the other panels: B. From 11.4 to 8.0. C. From 6.6 to 5.5. D. From 3.4 to 3.3.
The H-NMR spectrum (Fig. 4A, B) showed signals indicative of a formyl group (11.17 ppm) and hydrogens positioned on the alpha (C5), beta (C10) and delta (C20) carbons of the tetrapyrrol cycle (9.49, 9.37 and 8.55 ppm, respectively). The spectrum also showed four signals (6.6-6.1 and 5.7-5.5 ppm) coupled with a signal in 8.0 ppm indicating the presence of a vinyl group (Fig. 3A, C). In addition, two signals at 3.4 and 3.2 were observed, indicating hydrogens belonging to two methyl groups on C1 and C12 (Fig. 4A, D). These observations were integrated in the proposed formulae (Fig. 5) which correpond to pheophorbides a and b.
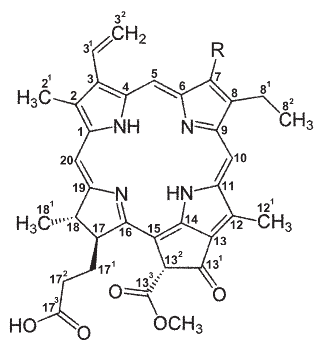
Figure 5. Proposed structure of the major green pigments present in the CII fraction of acetone extracts of the C endosymbiont. Either a methyl or a formyl group occurs at position 7 (denoted by R) in pheophorbides a or b, respectively.
The smaller green fraction (CIII) was also processed by H-RMN. It showed a similar hydrogen resonance pattern to that of the CII fraction, but the formyl group in the C7 position was replaced by a methyl group. Other signals could not be assigned but the pigment was tentatively identified as a modified pheophorbide b with some polar substituent/s which decreased its chromatographic mobility.
Ligth absorption spectra of aqueous extracts of C corpuscles and Nostoc sp.
The absorption spectra (400 and 700 nm) of an aqueous extract of C endosymbiont (continuous line) and Nostoc sp. (dashed line) are shown in Fig. 6. The values are expressed as percent of the maximum absorbance value in each case. Nostoc sp. showed the highest absorption between 550 and 650 nm (peak at 615 nm). The extract from the C endosymbiont showed very low absorption, but the percent of the maximum was higher than 50% between 550 and 650 nm, and a single small peak was observed at 670 nm. This light absorption pattern may be explained by (1) the lack of phycobiliproteins, and (2) the presence of small amounts of a polar pheophorbide as that of fraction CIII of acetone extracts.

Figure 6. Ligth absorption spectrum of an aqueous extract of the C endosymbiont isolated from the midgut gland of P. canaliculata (continuous line) and of an axenic culture of Nostoc sp. (dashed line). Results were expressed as percent of the highest absorbance value in each case.
Fluorescence emission spectra and protein electrophoresis
The fluorescence emission spectra of aqueous extracts of C corpuscles (continuous lines) and Nostoc sp. (dashed lines) were obtained after excitation with wavelengths suitable for C-phycocyanin (618 nm) and allophycocyanin (647 nm). The Nostoc sp. extract showed fluorescent emissions at 615 and 652 nm, as expected for the corresponding phycobiliproteins (Fig. 7A, B; dashed line); sensitivity of detection was 1.4 and 14 µg protein/mL for C-phycocyanin and allophycocyanin, respectively). However, no fluorescent emissions occurred when the aqueous extracts of the C endosymbiont were excited with the same wavelengths (Fig 7A, B; continuous line), in a protein concentration range of 1.5-465 µg/mL.

Figure 7. A. Fluorescence excitation for C-phycocyanin (618 nm, gray line) and the resulting emission spectrum of aqueous extracts of C endosymbionts (continuous lines) and Nostoc sp. (dashed lines). B. Fluorescence excitation for allophycocyanin (647 nm, gray line) and the resulting emission spectrum in aqueous extracts of C endosymbionts (continuous lines) and Nostoc sp. (dashed lines). C. Protein electrophoresis (SDS-PAGE 15%) of aqueous extracts of C endosymbionts and Nostoc sp. (14 µg of soluble protein were loaded in each lane). MW= markers of apparent molecular weight.
Also, no fluorescence emission corresponding to phycoerithrin (575 nm) was detected in aqueous extracts of either C endosymbionts or Nostoc sp. cells (data not shown in Fig. 7).
The protein moieties of phycobiliproteins in both aqueous extracts were separated SDS-PAGE as shown in Fig. 7C. The extract from Nostoc sp. showed two bands of 19.2 and 16.1 kDa corresponding to the protein moieties of both studied phycobiliproteins (Mac Coll, 1998), while the extract of the C endosymbiont only showed some bands larger than 50 kDa.
Discussion
The nature of the intracellular endosymbiont/s in the midgut gland of Pomacea canaliculata is still an unresolved matter. We have previously hypothesized that it would be an endocyanobiont (Vega et al., 2006). However, we have recently found (Vega and Dellagnola, unpublished) a striking similarity of the 16S rRNA gene sequence found in C corpuscles with that of plant chloroplasts. These findings should not be interpreted plainly as to identify the C endosymbiont with a chloroplast, since several observations point into other directions, namely, (1) C corpuscles exhibit an electron dense wall that chloroplasts do not have (Vega et al., 2005; Koch et al., 2006); (2) they are directly transferred from the mother to the offspring (Koch et al., 2003); and (3) they are exocytized towards the gut lumen and expelled in the feces to the environment, where they are found for months or even years in sediments of aquaria or in environments formerly occupied by snails (Koch et al., 2006; Vega et al., 2006).
1. Chlorophylls a and b, and their pheophorbides.
Chlorophyll a is the most abundant chlorophyll throughout the kingdom Plantae (which includes chlorophyte algae, stoneworts, mosses, ferns, gymnosperms and angiosperms) (Raven et al., 2005) and is also found in the Cyanobacteria (Lee, 2008). All oxygenic photosynthetic organisms utilize chlorophyll a but differ in other pigments like chlorophyll b, which is also found throughout the kingdom Plantae but not in the Cyanobacteria, with the exception of the Prochlorales, oxygenic-photosynthetic bacteria which also show chlorophyll b, but that are now frequently included within the Cyanobacteria based on their molecular affinities (Lewin, 2002; Litvaitis, 2002).
Indeed, not the chlorophylls but the corresponding pheophorbides a and b (and also a modified, and more polar, pheophorbide a) have been found in the C endosymbiont. Pheophorbides are not functional for light harvesting but are the result of chlorophyll breakdown initiated by chlorophyllase, a process closely regulated throughout the entire life cycle of plants (Hörtensteiner, 2006), and which is induced in many cases by light deprivation (e.g., Wittenbach, 1977). This may occur in the C endosymbiont, which should be regarded as a potentially phosynthetic organism or plastid that is kept in darkness, at least when contained within cells of the midgut gland, an internal organ of the snail which is also covered by the melanic mantle epithelium and the shell (Dellagnola, 2010).
Chlorophyll breakdown has also the functional significance of preventing the potential damage resulting from the photodynamic activity of free chlorophylls (Goldschmidt, 2000). So, it is possible that both chlorophylls are synthesized by the C endosymbiont, and that they are largely converted to the corresponding pheophorbides. The existence of the biosynthetic machinery for both chlorophylls tends to support the affinity of C corpuscles with plant chloroplasts.
2. Phycobilins
No evidence of either C-phycocyanin, allophycocyanin or phycoerithrin was found in C corpuscles, which argues against a cyanobacterial affinity of the endosymbiont. Again, one may wonder whether the expression of these pigments in C corpuscles was reduced by darkness. However, no evidence of phycobiliproteins was obtained when the concentration of the sample was increased more than 300-fold.
3. A sterol
The CI fraction of the acetone extract of C corpuscles contained a substance bearing some similarities with cholesterol. Cholesterol and other sterols are well-known normal constituents of plant chloroplast membranes (Grunwald, 1975) and so, the presence of a sterol also argues in favor of the affinity of the C endosymbiont with plant chloroplasts. However, sterols have also been reported in Cyanobacteria (De Souza and Nes, 1968; Kohlhase and Pohl, 1988) but later studies have questioned their presence because of possible contaminations of cyanobacterial cultures (Volkman, 2003; Volkman, 2005). So, at the present state of knowledge, the current results do not shed any light on the problem of the phylogenetic affinities of C corpuscles.
4. Concluding considerations
In general, the present work provides suggestive evidence for the affinity of the C endosymbiont of P. canaliculata with plant chloroplasts, rather than with Cyanobacteria.
A peculiar symbiotic consortium involves marine slugs (Opistobranchia, Elysiidae) and chloroplasts, which the slugs obtain from chlorophyte or xanthophyte algae. This symbiosis is referred to as ‘kleptoplasty' (Gilyarov, 1983). The chloroplasts involved are dietary chloroplasts incorporated intracellularly in numerous terminal tubules of the midgut gland, which are located below the slug's transparent dorsal mantle (Kawaguti and Yamasu, 1965; Taylor, 1968; Trench et al., 1969) and are able to photosynthetically reduce carbon when the slug is exposed to light, and to transfer photosynthate to their host (Rumpho et al., 2000).
As discussed elsewhere (Vega et al., 2006) kleptoplasty shows interesting parallels with the symbiosis studied here. However, three major differences stand out: (1) while the symbiotic chloroplasts are dietary ones, and are continuously incorporated by the slugs to their cells, the symbiont of P. canaliculata and other Neotropical Ampullaridae (Castro-Vazquez et al., 2002) is vertically transferred from the mother to the offspring (Koch et al., 2003) and so, the original association should have occurred (perhaps only once) between the ancestor of Neotropical Ampullariidae and the chloroplast of an aquatic macrophyte; (2) while chloroplasts associated to slugs are phosynthetically active, the pheophorbides present in C corpuscles (this paper) as well as their location in an intracellular dark environment (Dellagnola, 2010) do not support this possibility; (3) the C endosymbiont shows features of an organism (i.e., its external wall, and its ability to survive outside the snail; Koch et al., 2006). So, it is still possible that the ancestral association may have occurred with a free living prokaryont akin to the Cyanobacteria and plastids.
Finally, if the C endosymbiont is unable to participate in photosynthesis, one may wonder which is the functional meaning of its association with P. canaliculata, especially if one considers the extent to which the midgut gland is occupied by the symbiotic corpuscles (Vega et al., 2005). Recent evidence indicates that they may at least participate in metal detoxification (Vega et al., 2012) and in protein digestion in the snail's gut (Godoy, 2005).
Acknowledgements
The authors thank Professor M.L. Tomaro for the use of facilities. This work was supported by grants from FONCyT, CONICET and the National University of Cuyo.
References
1. Bennett A, Bogorad L (1973). Complementary chromatic adaptation in a filamentous blue-green alga. The Journal of Cell Biology 58: 419-435. [ Links ]
2. Castro-Vazquez A, Albrecht EA, Vega IA, Koch E, Gamarra-Luques C (2002). Pigmented corpuscles in the midgut gland of Pomacea canaliculata and other Neotropical apple-snails (Prosobranchia, Ampullariidae): A possible symbiotic association. Biocell 26: 101-109. [ Links ]
3. Cowie R (2002). Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. In: Molluscs as crop pests: (Baker, GM, ed), p. 145-192. CABI, Wallingford, UK. [ Links ]
3. Cowie R (2002). Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. In: Molluscs as crop pests: (Baker, GM, ed), p. 145-192. CABI, Wallingford , UK.
4. de Souza NJ, Nes WR (1968). Sterols: isolation from a blue-green alga. Science 162: 363-364. [ Links ]
5. Dellagnola FA (2010). Una simbiosis procariota-molusco: Contribuciones funcionales y taxonómicas. License Degree Dissertation. Instituto de Ciencias Básicas, Universidad Nacional de Cuyo. [ Links ]
6. Gilyarov M (1983). Appropriation of functioning organelles of food organisms by phytophagous and predatory opisthobranch mollusks as a specific category of food utilization. Zhurnal Obshchei Biologii 44: 614-620. [ Links ]
7. Godoy M (2005). Proteasas de la glándula digestiva de Pomacea canaliculata (Caenogastropoda, Ampullariidae), y su relación con una bacteria endocitobiótica. License Degree Dissertation. Universidad Nacional de San Luis. [ Links ]
8. Goldschmidt EE (2000). Chlorophyll decomposition in senescing leaves and ripening fruits: Functional and evolutionary perspectives. ISHS Acta Horticulturae 553: 331-336. [ Links ]
9. Grunwald C (1975). Plant sterols. Annual Review of Plant Physiology 26: 209-236. [ Links ]
10. Halwart M (1994). The golden apple snail Pomacea canaliculata in Asian rice farming systems: present impact and future threat. International Journal of Pest Management 40: 199-206. [ Links ]
11. Hayes KA, Joshi RC, Thiengo SC, Cowie RH (2008). Out of South America: multiple origins of non native apple snails in Asia. Diversity and Distributions 14: 701-712. [ Links ]
12. Hörtensteiner S (2006). Chlorophyll degradation during senescence. Annual Review of Plant Biology 57: 55-77. [ Links ]
13. Kawaguti S, Yamasu T (1965). Electron microscopy on the symbiosis between an elysioid gastropod and chloroplasts of a green alga. Biological Journal of the Okayama University 11: 57-65. [ Links ]
14. Koch E, Vega I, Albrecht E, Gamarra-Luques C, Castro-Vazquez A (2003). Evidence for direct mother-offspring transmission of a possible symbiont inhabiting the midgut gland (MGG) of Pomacea canaliculata. Biocell 27: 24 (abstract). [ Links ]
15. Koch E, Vega IA, Albrecht EA, Ortega H, Castro-Vazquez A (2006). A light and electron microscopic study of pigmented corpuscles in the midgut gland and feces of Pomacea canaliculata (Caenogastropoda: Ampullariidae). Veliger 48: 17-25. [ Links ]
16. Kohlhase M, Pohl P (1988). Saturated and unsaturated sterols of nitrogen-fixing blue-green algae (cyanobacteria). Phytochemistry 27: 1735-1740. [ Links ]
17. Lee RE (2008). Phycology. Cambridge University Press. [ Links ]
18. Lewin RA (2002). Prochlorophyta-a matter of class distinctions. Photosynthesis research 73: 59-61. [ Links ]
19. Litvaitis M (2002). A molecular test of cyanobacterial phylogeny: inferences from constraint analyses. Hydrobiologia 468: 135-145. [ Links ]
20. Lowry O, Rosebrough N, Farr A, Randall R (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265-275. [ Links ]
21. MacColl R (1998). Cyanobacterial phycobilisomes. Journal of Structural Biology 124: 311-334. [ Links ]
22. Raven PH, Evert RF, Eichhorn SE (2005). Biology of Plants. W.H. Freeman. [ Links ]
23. Rawlings TA, Hayes KA, Cowie RH, Collins TM (2007). The identity, distribution, and impacts of non-native apple snails in the continental United States. BMC Evolutionary Biology 7: 97. [ Links ]
24. Rumpho ME, Summer EJ, Manhart JR (2000). Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis. Plant Physiology 123: 29-38. [ Links ]
25. Sambrook J, Fritsch E F, Maniatis T (1989). Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York. [ Links ]
26. Taylor D (1968). Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis [Gastropoda: opisthobranchia]. Journal of the Marine Biological Association of the UK 48: 1-15. [ Links ]
27. Trench RK, Greene RW, Bystrom BG (1969). Chloroplasts as functional organelles in animal tissues. The Journal of Cell Biology 42: 404-417. [ Links ]
28. Vega IA, Gamarra-Luques C, Koch E, Bussmann L, Castro-Vazquez A (2005). A study of corpuscular DNA and midgut gland occupancy by putative symbiotic elements in Pomacea canaliculata (Caenogastropoda, Ampullariidae). Symbiosis 39: 37-45. [ Links ]
29. Vega IA, Damborenea MC, Gamarra-Luques C, Koch E, Cueto JA, Castro-Vazquez A (2006). Facultative and obligate symbiotic associations of Pomacea canaliculata (Caenogastropoda, Ampullariidae). Biocell 30: 367-375. [ Links ]
30. Vega IA, Arribére MA, Almonacid AV, Ribeiro Guevara S, Castro-Vazquez A (2012). Apple snails and their endosymbionts bioconcentrate heavy metals and uranium from contaminated drinking water. Environmental Science and Pollution Research, DOI 10.1007/S11356-012-0848-6. [ Links ]
31. Volkman J (2003). Sterols in microorganisms. Applied microbiology and Biotechnology. 60: 495-506. [ Links ]
32. Volkman JK (2005). Sterols and other triterpenoids: source specificity and evolution of biosynthetic pathways. Organic Geochemistry 36: 139-159. [ Links ]
33. Wittenbach VA (1977). Induced senescence of intact wheat seed-lings and its reversibility. Plant Physiology 59: 1039-1042. [ Links ]
Revised version received: March 10, 2011.
Accepted: November 23, 2011.














