Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Biocell
versión impresa ISSN 0327-9545
Biocell vol.36 no.3 Mendoza dic. 2012
BRIEF NOTE
Pollen viability of Polygala paniculata L. (Polygalaceae) using different staining methods
Viviane Dal-Souto Frescura1, Haywood Dail Laughinghouse IV2, Thais Scotti do Canto-Dorow1, Solange Bosio Tedesco1*
1 Laboratory of Cytogenetics and Genotoxicity, Department of Biology, Centro de Ciências Naturais e Exatas (CCNE), Universidade Federal de Santa Maria (UFSM), Avenida Roraima, nº 1000, Cep. 97105-900, Camobi, Santa Maria, Rio Grande do Sul, Brasil.
2 Centre for Protein Engineering, Institute of Chemistry B6, University of Liège, Belgium.
*Address correspondence to:
Solange Bosio Tedesco. E-mail: solatedesco@yahoo.com.br
ABSTRACT: Polygala paniculata L. is a medicinal plant that grows in the Brazilian Atlantic coast, known as 'barba-de-São-João', 'barba-de-bode', 'vassourinha branca', and 'mimosa'. In this study, pollen viability was estimated by three different staining methods: 2% acetic orcein, 2% acetic carmine, and Alexander's stain. The young inflorescences of twenty accessions were collected and fixed in a solution of ethanol: acetic acid (3:1) for 24 hours, then stored in ethanol 70% under refrigeration. Six slides per plant, two for each stain, were prepared by squashing, and 300 pollen grains per slide were analyzed. Pollen viability was high (>70%) for most accessions of P. paniculata using the Alexander's stain, which proved the most adequate method to estimate pollen viability.
Key words: Pollen grains; Barba-de-bode; Acetic orcein; Acetic carmine; Alexander's stain.
Polygala paniculata L. ('barba-de-São-João', 'barba-de-bode', 'vassourinha branca', or 'mimosa') is frequently found on the Brazilian Atlantic coast, and it is used in traditional medicine for treating asthma, chronic bronchitis, arthritis, osteoarthritis, kidney diseases, stomach aches and diarrhea (Newall et al., 1996). Some compounds have been isolated from P. paniculata, and identified as xanthones, coumarins, the flavonoid rutin, and phytosterols (Cristiano et al., 2003).
Estimation of pollen viability is important for the analysis of gene flow in plants, because it evidences the masculine breeding potential of the species and may be useful in taxonomic, ecological, genetic and palynological studies. The Alexander's stain bases the distinctionbetween viable and non-viable grains on the differential staining of the protoplasm and the cellulose containing wall (Alexander, 1980). Also, Paula (2009) suggested that the acetic orcein and acetic carmine stains can overestimate the viability of the pollen grains, because they stain both viable and non-viable pollen the same color (red), and only show differences in intensity.
The aim of the study was to evaluate pollen grain viability of P. paniculata accessions using three staining methods. The plant material was collected in the municipality of Santa Maria, Rio Grande do Sul, Brazil, at the beginning of flowering. Pollen viability from 20 accessions was evaluated using 2% acetic orcein, 2% acetic carmine, and Alexander's stain (malachite green and acid fuchsin).
Plant inflorescences were harvested and fixed in ethanol: acetic acid (3:1) at room temperature for 24 hours. They were then kept refrigerated in 70% ethanol until preparation of slides by the squashing technique (Guerra and Souza, 2002).
In the cases of 2% acetic orcein and 2% acetic carmine solution, the viability was determined directlyby the difference in size and staining capacity of pollen grains. Grains that presented darker tonalities and were larger in size were considered viable. In the case of Alexander's stain, the material was covered with a coverslip after staining, sealed with adhesive, stored in a refrigerated environment and, the slides were analyzed after 24 hours. Only large, purple-colored pollen grains were considered viable, while smaller, blue-green colored grains were considered non-viable.
Six slides per plant were evaluated, two for each stain, using light microscopy (400x) and 300 grains were analyzed for each slide. Results were expressed as percent of viable grains.
Statistical analysis was performed using Assistat®, version beta 7.5. The data were subjected to analysis of variance and the means were compared by the Tukey test at 5% (P < 0.05).
This study showed that 2% acetic orcein (Fig. 1A) did not allow discrimination in regards to the estimation of pollen grain viability in most of the studied accessions (Table 1). This stain showed the highest pollen viability (98.9%, Table 1) which was a likely overestimation due to the difficulty in distinguishing among stain tonalities and so, evaluation relied mainly on differences in size (Fig. 1A).
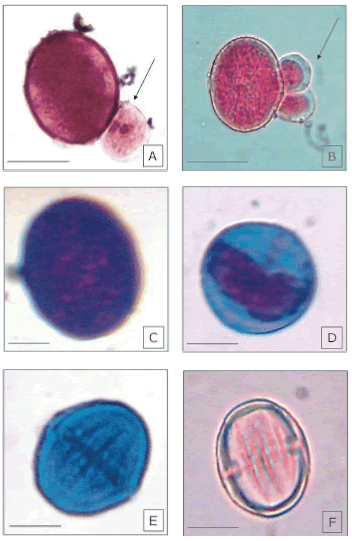
Figure 1. Pollen grains stained with 2% acetic orcein, 2% acetic carmine, and Alexander's stain. A. Pollen grains stained with 2% acetic orcein; the arrow indicates a small, non-viable pollen grain. B. Viable pollen, stained with 2% acetic carmine; the arrow indicates two small, non-viable pollen grains. C. Viable pollen grain stained with the Alexander's stain. D and E are non-viable pollen grains stained with Alexander's stain. F. Non-viable pollen grain stained with 2% acetic carmine. Scales represent 10 µm.
Table 1. Mean pollen viability (%) for each of 20 accessions of Polygala paniculata stained with 2% acetic orcein, 2% acetic carmine, and Alexander's stain

In 2% acetic orcein studies of pollen viability in populations of the medicinal plant species Aloysia gratissima ('alfazema-do-Brasil') and Aloysia triphylla ('cidró') Hister et al. (2010) also observed very high viability values (>97%).
The use of 2% acetic carmine allowed more discrimination, since both different tonalities and sizes of the pollen grains could be studied (Fig. 1 B and F). This method yielded 80.4% viability in P. paniculata (Table 1).
On its part, the Alexander's stain yielded 72.3% viability in P. paniculata (Table 1), differing significantly from the other stains used, and appeared in this study as that more able to discriminate between viable and non-viable pollen grains.
The estimation of pollen viability by the Alexander's stain appears to provide more accurate data, because of the contrasting colors resulting from malachite green and acid fuchsin staining. The protoplasm of viable pollen grains becomes purple with acid fuchsin (Fig. 1 C), while the non-viable pollen grains only show a poorly distributed (or absent) purple protoplasm and the blue-green cellulosic wall (Fig. 1 D and E) (Alexander, 1980).
Auler et al. (2006), using either 2% propionic carmine, 2% acetic orcein, or the Alexander's stain in the medicinal species Baccharis trimera ('carqueja'), also concluded that the Alexander's stain was the best to estimate pollen viability.
In P. paniculata, pollen viability as determined with the Alexander's stain showed high values (>70%) for most of the accessions (1, 3, 4, 5, 6, 8, 9, 11, 12, 13, 14, 15, 19 and 20, Table 1). However, some accessions showed viability values lower than 70% (2, 5, 7, 10,16, 17 and 18, Table 1). These differences may indicate possible meiotic irregularities provoking different degrees of sterility, as observed by Bione et al. (2005) in soybeans.
It is concluded that P. paniculata has a high pollen viability, and that this suggest the feasibility of programs for genetic improvement in this species.
References
1. Alexander MP (1980). A versatile stain for pollen, fungi, yeast and bacteria. Stain Technology 55: 13-18. [ Links ]
2. Auler NMF, Battistin A, Reis MS (2006). Número de cromossomos, microsporogênese e viabilidade do pólen em populações de carqueja [Baccharis trimera (Less.) DC.] do Rio Grande do Sul e Santa Catarina. Revista Brasileira de Plantas Medicinais 8: 55-63. [ Links ]
3. Bione NCP, Pagliarini MS, Almeida LA (2005). A male-sterile mutation in soybean (Glycine max) affecting chromosome arrangement in metaphase plate and cytokinesis. Biocell 29: 177-181. [ Links ]
4. Cristiano R, Pizzolatti MG, Delle FM, Rezende CM, Branco A (2003). Two xanthones from Polygala paniculata and confirmation of the 1-hydroxy-2,3,5-trimethoxy-xanthone at trace level by HRGC-MS. Zeitschrift für Naturforschung 58: 490-494. [ Links ]
5. Guerra M, Souza MJ (2002). Como observar cromossomos - Um guia de técnicas em citogenética vegetal, animal e humana. Funpec, Ribeirão Preto. [ Links ]
6. Hister CAL, Tedesco SB, Ferreira AC, Canto-Dorow TS (2010). Meiotic behavior and pollen viability of Aloysia gratissima and Aloysia triphylla (Verbenaceae). Ciência e Natura 32: 37-47. [ Links ]
7. Newall AC, Anderson AL, Phillipson DJ (1996). Herbal Medicines: A guide for health-care professionals. The Pharmaceutical Press, London. [ Links ]
8. Paula JM (2009). Caracterização e manejo de Conyza spp. resistente ao herbicida glifosato. Dissertação (Mestrado em Fitossanidade) Curso de Pós-Graduação em Fitossanidade, Universidade Federal de Pelotas. [ Links ]
Received: June 24, 2011.
Revised version received: August 31, 2012.
Accepted: September 11, 2012.














