Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Anales de la Asociación Química Argentina
versión impresa ISSN 0365-0375
An. Asoc. Quím. Argent. v.93 n.1-3 Buenos Aires ene./jul. 2005
ELECTRONIC NOSES APPLICATIONS AND TECHNOLOGIES
A thin film sensor to detect ammonia at room temperature in humid mediaBianchetti1, M. F.; Heredia2, E.; Oviedo3, C.; Walsöe de Reca4, N. E.
CONICET1, CITEFA2, CNEA-CIC3, CONICET-CITEFA4
CINSO (Centro de INvestigaciones en SOlidos) CITEFA-CONICET
Juan Bautista de La Salle 4397, Villa Martelli (B1603ALO) Buenos Aires, Fax: (54) (11)-4709-8241, walsoe@citefa.gov.ar4 ;mbian@citefa.gov.ar1.
Received November 05, 2004. In final form May 19, 2005
Abstract
A thin film sensor for soils to detect ammonia in aqueous media, operating at room temperature, is reported. The sensor was built by depositing pure tellurium by thermal evaporation in conventional vacuum (10-6 Hg mm) on electronic purity alumina substrates fitted with gold electrodes. The resistance of tellurium films was measured when the sensor was exposed to ammonia in humid air. Films were characterized by using the following techniques: Scanning Electron Microscopy (SEM) to observe films morphology, X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS) to look for possible surface tellurium oxides. In every case, specimens were observed or analyzed in its initial state and after being aged in different atmospheres: humidity, air and (ammonia + humidity). The appearance of surface TeO2 in Te specimens aged in air, enabled to infer a probable sensing mechanism of NH3 in humid media.
Resumen
Se informa sobre un sensor de película delgada para detectar amoníaco en medio húmedo en suelos, operable a temperatura ambiente. El sensor fue construido depositando telurio puro por evaporación térmica en vacío convencional (10-6 mm Hg) sobre sustratos de alúmina de pureza electrónica provistos con electrodos de oro. La variación de la resistencia de las películas de telurio se midió cuando el sensor era expuesto a amoniaco en aire húmedo. La superficie de las películas se caracterizó empleando las siguientes técnicas: Microscopía Electrónica de Barrido (SEM) para observar la morfología de las películas, Difracción de Rayos X (XRD) y Espectroscopía de Fotoelectrones (XPS) para buscar posibles óxidos de telurio superficiales. En cada caso, las muestras fueron observadas o analizadas en su estado inicial o después de habérselas envejecido por exposición a diferentes atmósferas: humedad, aire y (amoníaco + agua). La presencia de TeO2 superficial en muestras de Te envejecidas en aire permitió inferir un mecanismo probable de sensado de NH3 en medio húmedo.
Introduction
It has been found that tellurium-based thin films exhibited a high sensitivity to nitrogen dioxide at room temperature detecting a concentration as low as 3 ppm of this pollutant and toxic gas released by combustion industries and vehicles [1]. The resistance of tellurium films changes reversibly in the presence of NO2. Besides, the same authors [2] investigated the effect of propylamine (C3H7NH2) and other aliphatic amines on the electrical conductivity of tellurium thin films at room temperature.
A sensor to detect ammonia in aqueous media for soils (agricultural use) was required to the authors of this paper, pointing out the necessity of room temperature operation and a long working life. Several NH3 and NO2 sensors were built, employing semiconductor oxides (like SnO2 or WO3) but with an operating temperature always in the range 350°C and 450°C [3-5]. Also polymer thin film sensors have been reported [6] but their manufacture was not reproducible and they exhibited a variable response in the presence of humidity. When the present work was in progress another interesting paper was published on sensing properties of tellurium films for ammonia in dry atmosphere [7].
Experimental Procedure
Thin tellurium films were grown by thermal vacuum (10-6 Hg mm) evaporation of pure tellurium in an E306 Edwards equipment on electronic purity alumina substrates (1 cm x 2 cm) maintained at 150° C. Films, 180 to 200 nm thick, were grown in order to control de grain size. Films were studied by X-Ray diffraction (XRD) with a Philips PW 3710 diffractometer, employing the Cu-Ka radiation. Crystallite size was determined with Scherrer equation: D = 0.9 l / b cosq, where: D is the crystallite size, l is the wave length of the incident radiation (1.5418 Å for Cu-Ka radiation), b is the full width half maximum (FWHM) of the peak and q is the peak position. The instrument width, necessary to correct b, was evaluated by deposition of an Al2O3 film with a mean crystallite size of 25 mm.
Figure 1: X-ray diffractogram of a Te polycrystalline film exposed to air for five days, * indicates the Te peaks.
Figure 1 is the polycrystalline film X-ray diffractogram in which only peaks corresponding to pure tellurium are observed. No peaks corresponding to surface tellurium oxides were shown by XRD pattern, probably, because oxide films were extremely thin. Consequently, XPS measurements were performed. No-preferred orientations of Te film crystals could be detected by XRD analysis.
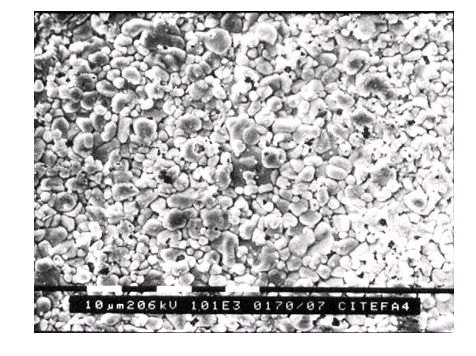
Figure 2: SEM micrograph (M = 1000x) of a Te film after being exposed to air for five days.

Figure 3: SEM micrograph of a magnified zone (M = 10000x) of the Te film of Figure 2.
Films were observed by SEM with a 505 Philips equipment. Figure 2 shows the micrograph of a Te film exposed for five days to air, taken with a magnification M= 1000x. Figure 3 is a higher magnification of a zone of the previous specimen (M=10000x) showing little crystals of probable tellurium oxides grown on the surface of tellurium grains Figure 4 is the micrograph of a Te film in contact with humid atmosphere for fourteen days (M= 10000x). Otherwise, Te films exposed to the gas in thermal equilibrium with a solution of 5% NH3 in water for five days, showed a completely different morphology as damaged by a strong corrosion, Figure 5 (M=10000x).

Figure 4: SEM micrograph of a Te film exposed to humid atmosphere for 15 days (M = 10000x)

Figure 5: SEM micrograph of Te film exposed to the gas in thermal equilibrium with a solution of 5 % NH3 in water, for five days (M = 1000x).
Gold was evaporated on films ends to build the sensor and silver terminals were attached to the evaporated layer with silver painting after verifying that these contacts were not rectifying. The mean initial resistivity of films resulted 1.27. 10-1 W.cm as measured with the four-point method with a HP 3469 multimeter (provided with an external power source).
Relative resistance was measured with a 617 Keithley electrometer and plotted versus the ammonia concentration in humid air. Measurements were carried out thermostated at 20°C medium with a K4R Lauda Thermostat with a R20K regulator in containers with solutions of NH3 (50000 ppm, 25000 ppm, 12500 ppm, 6250 ppm, 625 ppm and 63 ppm) in water. At higher NH3 concentrations, the sensors resulted completely damaged. To appreciate the real sensitivity of sensors, these measurements were made at lower NH3 concentrations in humid atmospheres.
Results and Discussion
Figure 6 shows the surface resistance variation (DR in kW) versus ammonia concentration (ppm) in humid air measured as described above. It was also measured the resistance variation in successive cyclings in dry air, humid air and air with 500 ppm of NH3, plotting the resistance [R in kW] versus the time [t in s]. Finally, the sensor sensitivity S = R(NH3-H2O)/R(H2O) [where R(NH3-H2O): resistance in humid air with 500 ppm of NH3 and R(H2O): resistance in humid air] was plotted versus time [t in s], Figure 7, for successive cyclings in air, humid air and (NH3 + humid air) each cycling being indicated with a number.
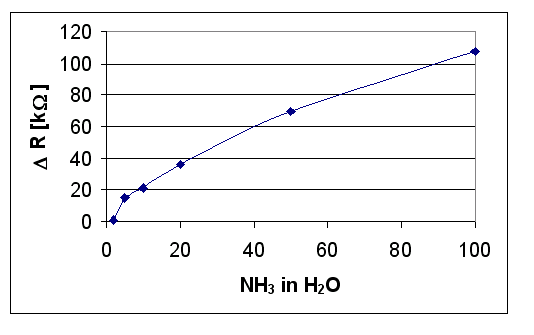
Figure 6: DR (in kW) versus NH3 concentration (in ppm).
XPS studies were conducted in order to determine the chemical composition of films surface. Three sets of tellurium specimens were vacuum deposited on silver sheet substrates under the above described conditions, specimens of one set aged in air [air], those of the second in humid atmosphere [H2O] and specimens of the third set in a solution of 5% NH3 in water [NH3].
XPS measurements were carried out using the MgKa line (1253.6 eV) as incident radiation. The binding energies (B.E.) were calibrated considering the Au 4f7/2 peak at 83.9 eV with respect to the Fermi level. In every case, a wide spectrum in a 0 to 1100 eV energy range was taken to determine the elements present on the specimens surface, without previous A+ ion cleaning. The three types of specimens exhibited wide spectra with XPS peaks of Te, C, O, Ag and Auger peaks of Ag and Te. As the binding energy (BE) of the most important XPS peak of Te3d5/2 was coincident with the B.E. of the Ag3p3/2 peak and, taking into account that due to the specimens size it was impossible to avoid the substrate signal (Ag), the Te 4d peak was also measured as a reference. Narrow spectra O1s, Te3d and Te4d were taken with energy high resolution (Epass=20 eV). Figure 8a shows the O1s spectra and Figure 8b the Te3d spectra of the three specimen types. The O1s peaks of [H2O] and [NH3] specimens correspond mainly to physically adsorbed O2/H2O (B.E.: 532.3±0.2 eV) and the energy position for [air] specimens (B.E.: 530±0.2 eV) was in the metallic oxides range [8, 9]. The Te3d5/2 spectra of [H2O] and [NH3] with B.E. of 572.3±0.1eV, corresponds to pure tellurium with the Ag contribution. The sample [air] presents this peak and also a signal with B.E. of 575.5±0.2 eV which is attributed to TeO2 [9]. It is possible that films were oxidised in air by reaction of Te with O2, producing a thin tellurium oxide coating on Te grains.

Figure 7: Sensitivity S = R(NH3-H3O)/R(H2O) versus time [t in s] for successive cyclings, each cycling being indicated with a number.
Taken into account these results, it is possible to accept that the oxidized Te film behaves as a p-semiconductor exhibiting a space charge [Te+TeO2+physically adsorbed oxygen] surrounding the Te grains and, particularly, the grain boundaries. Consequently, it is possible to assume that adsorbed oxygen atoms can trap electrons increasing the number of holes, not only at the grain boundaries but also at the grains surface, causing an increase of conductivity in both regions. But, in spite of the high holes density, this increase is small because of the high defects density and by the presence of TeO2. If films are exposed to an (NH3 + H2O) atmosphere, the removal of adsorbed oxygen decreases the majority carriers density and the resistivity of films increases as it was observed.
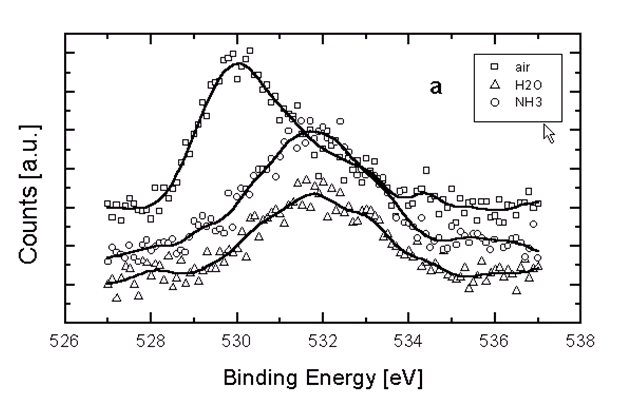

Figures 8a and 8b: XPS spectra of three specimen types: [air], [H2O] and [NH3+H2O]; a) O1s; b) Te3d.
Conclusions
Properties of vacuum evaporated Te films were studied in order to use them for an NH3 humid media sensor for agricultural application in soils. The sensor was showed to be sensitive to (NH3 + H2O) atmosphere, operating at room temperature. As it was observed by SEM that Te films were partially or totally covered with thin films of small crystals, it was assumed that Te films were oxidized by exposition to air. XRD did not reveal the presence of oxides, probably due to the small thickness of oxide layers but, TeO2 was identified by XPS for specimens exposed to air. On the basis of XPS data, a possible mechanism of the effect of NH3 in humid media on Te films aged in air is proposed to explain the resistance increase measured in this work.
Acknowledgements
The authors are indebted to ANPCyT by PICT 8688-2000, “Functional Materials for Gas Sensors: Synthesis, Characterization and Applications”.
References
[1] Tsiulyanu, D.; Marian, S.; Miron, V.; Liess, H.–D., Sensors and Actuators, B-Chemical, 2001, 73, 35-39. [ Links ]
[2] Tsiulyanu, D.; Marian, S.; Liess, H.-D., Sensors and Actuators, B-Chemical, 2002, 85, 232-238. [ Links ]
[3] Sberveglieri, G.; Depero, L.; Groppelli, S.; Nelli, P., Sensors and Actuators, B-Chemical, 1995, 26-27, 89-94. [ Links ]
[4] Takao, Y.; Miyazaki, K.; Shimizu, Y.; Egashira, M. J. Electrochem. Soc., 1994, 141, 1028-1032. [ Links ]
[5] Marquis. B. T.; Vetelino, J. F., Sensors and Actuators, B-Chemical, 2001, 77, 100-1005. [ Links ]
[6] Christie, S.; Scorsone E.; Persaud, K.; Kvasnik, F., Sensors and Actuators, B-Chemical, 2003, 90, 163-167. [ Links ]
[7] Shashwati, S.; Muthe, K. P.; Niraj, J.; Gadkari, S. C.; Gupta, S. K.; Jagannath, M.; Roy, M.; Deshpande, S. K.; Yakhmi, J. V., Sensors and Actuators, B-Chemical, 2004, 98, 154-159. [ Links ]
[8] “Handbook of X-ray Photoelectron Spectroscopy”, Physical Electronics Inc., Ed. Chastain, J.; King, R., 1995. [ Links ]
[9] On-line Data Basis of “La Surface” (V. G. Scientific-France): www.lasurface.com. [ Links ]














