Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Anales de la Asociación Química Argentina
versão impressa ISSN 0365-0375
An. Asoc. Quím. Argent. v.93 n.4-6 Buenos Aires jan./dez. 2005
REGULAR PAPERS
N-(Phosphonomethyl) Glycine Interactions With Soils
Pessagno, R.C.1; Dos Santos Afonso, M.1; Torres Sanchez, R.M.2
1INQUIMAE and Departamento de Química Inorgánica, Analítica y Química Física - Facultad de Ciencias Exactas y Naturales - Universidad de Buenos Aires - Ciudad Universitaria, Pabellón II - (C1428EHA) Buenos Aires - Argentina. Fax: +54 11 4576 3341, E-mail: dosantos@qi.fcen.uba.ar
2CETMIC, CC 49, (B1896ZCA) M. B. Gonnet, Province of Buenos Aires, Argentina
Received July 29th, 2005. In final form August 22th, 2005
Dedicated to the memory of the late Prof. Hans J. Schumacher on the occasion of his 100th birthday
Abstract
Glyphosate is a non selective, broad spectrum, postemergent herbicide widely used in weed control. The adsorption isotherms and surface coverage of glyphosate (NPhosphonomethylglycine, PMG) in aqueous suspensions of Argentine soils as a function of PMG concentration and pH were measured. Zeta potential curves for the PMG/soils system were also determined. The formation of inner sphere surface complexes of PMG on the soil surface, were analyzed as a function of pH and surface coverage.
Resumen
Glifosato es un herbicida no selectivo, post-emergente y de amplio espectro, muy utilizado para el control de malezas. Las isotermas de adsorción y el cubrimiento superficial de glifosato (N-fosfonometilglicina, PMG) en suspensiones acuosas de suelos argentinos fueron medidas como una función de la concentración de PMG y del pH. También fueron determinadas las curvas de potencial zeta para el sistema PMG/suelo. La formación de complejos superficiales de esfera interna de PMG sobre la superficie de los suelos fue analizada como una función del pH y del cubrimiento superficial.
Introduction
Phosphonic acids and their derivatives are of interest due to their structural variety and great economic importance. Phosphonic acids derivatives are used as crop protection agents (weed control) in water treatment, in metal processing, and as flame proofing agents [1]. Phosphonates are molecules containing one or more groups R-PO(OH)2. The dibasic phosphonic acids are mostly weaker than phosphoric acid.
These compounds possess not only a very high ability to form strong complexes [2, 3] with transition metals in aqueous solution but also show a large affinity for the surface of aluminum and iron oxides. All these properties play a very important role in the fate and rate of transport of these compounds in the environment. N-phosphonomethylglycine (PMG) with an empirical molecular formula of C3H8NO5P is commonly known as glyphosate. Glyphosate is the active component of non-selective, postemergent and broad spectrum commercial herbicides widely used in agriculture [3]. It is a white odorless solid, with a molecular weight of 169.1 g mol-1. At 25°C the solubility of glyphosate in water is 1.16 wt%. This relatively low solubility is due to strong intermolecular hydrogen bonds in the crystal lattice. Glyphosate is practically insoluble in organic solvents, and the log octanol/water partition coefficient (log Kow) is -4.1. Glyphosate is very soluble in dilute bases and in strong acids, forming soluble salts. The monoanionic salts are used in the commercial herbicide formulations with glyphosate. The most common salts used in commercial products are the monoisopropylammonium (used in Roundup®) and the monotrimethylsulfonium (trimesium) salts [3].
The phosphonic acid group with a very stable C-P bond makes glyphosate extremely resistant to chemical hydrolysis, thermal decomposition and photolysis [4]. All the three acidbase groups in glyphosate have the ability to dissociate in aqueous solution and the pKa1, pKa2 and pKa3 values have been determined by different analytical methods such as potentiometric titration [5-9] or NMR [10,11].
Significant amounts of herbicides may eventually reach the soil and remain in the soil or be transported to other areas before their ultimate decomposition due to the different application methods and weather conditions.
It is known that PMG is immobilized upon contact with soils and clay minerals because of the formation of surface complexes with metal ions [12-21]. Mc Bride and Kung [22] first studied the adsorption of PMG onto goethite by transmission infrared spectroscopy measurements on dry films of the oxide. Even though strong bands appeared in the infrared frequency range of the phosphonate group, no band assignments were reported. Sheals et al. examined the structures of PMG onto goethite via spectroscopic techniques as a function of pH and band assignments were made [23]. Afterwards, structures of the surface complexes were proposed based on ATR-FTIR spectra of aqueous suspensions and their electrophoretic mobilities [24]. The role of carboxylic acid in the structures of the surface complexes of the herbicide with goethite was also discussed [24,25]. Adsorption experiments of different mono and polyphosphonates onto iron (III) hydr(oxide) [17] showed a striking similar adsorption behavior regardless of the different number of phosphonate moieties present in the ligand and the dissimilarity of their protonation level and net charge.
The focus of this study is to explain PMG adsorption on the surface of Argentine soils on the basis of the adsorption isotherms, XRD, zeta potential, surface speciation and previous results already reported [2,7,24,25].
Experimental
All solutions and suspensions were prepared using reagent grade chemicals and MilliQ water. Glyphosate (99 % pure) was a gift from Monsanto Argentina, Planta Zárate.
Goethite was prepared following the Atkinson Posner technique [26] by hydrolysis of ACS grade ferric nitrate with NaOH with a Fe/OH = 0.5 molar ratio. The solution was aged for two days at room temperature and then titrated to pH ~ 12.5 with NaOH. The resulting precipitate was aged for six more days at 60°C. The suspension was washed with Milli-Q water and dried in an oven at 40°C. Surface areas measured by N2 adsorption (BET analysis) were 60 m2/g for goethite. Suspensions of goethite were prepared by re-suspending the oxide in Milli-Q water and sonicating sporadically for two days to ensure hydration.
Soil samples from different provinces of Argentina were collected from different places. Ch, N, and A samples came from Chalten, Navaja, and Cerro Azul localities of Santa Cruz, Corrientes and Misiones provinces respectively. These soils were typified as Typic Haplocryoll, Udult and Rhodudults.
Soils were crushed and sieved using a < 2 mm sieve to remove plant residue and gravel and three times washed with distilled water. The iron oxide content was determined by chemical analysis. The mineralogical composition of the soils and the mineral purity were determined by XRD using a powder method on a Philips 3020 equipment with working conditions Cu Ka, 30 mA and 40 kV. The scanning was made at room temperature between 3° and 70° in 2θ with a step size of 0.02° and a step counting time of 2 sec. The quantitative analysis of the crystalline components was made using the Rietveld method [27] and profile matching mode [28] (see Table 1).
Table 1. Mineralogical composition of soils Q: Quartz + Cristobalite (SiO2), F: Feldspar, M: Montmorillonite, K: Kaolinite, H: Hematite + Magnetite (Fe2O3) and T: Anatase + Rutile (TiO2). Errors are indicated in parenthesis.

The IEP values were obtained by diffusion potential determination as described elsewhere [29]. Electromotive force (EMF) measurements were carried out with a Keithley 616 digital electrometer with a Metrohm calomel electrodes (Table 2).
Table 2. IEP, SN2 and Sw for Cerro Azul, Chalten, Navaja soils and goethite. * Data obtained from Reference [24], in this case ZPC was indicated instead of IEP. nd: none determined. Errors are indicated in parenthesis.

The total surface area of soil depends on both internal and external surface areas. Total specific surface area was measured using water adsorption method (Sw) [30]. External surface area was determined as normally by measuring the adsorption of N2 at temperatures near the boiling point of liquid nitrogen (SN2). Surface area analysis is based on the Brunauer, Emmett and Teller theory (BET method) (Table 2).
Suspensions of soils or iron oxides were brought to a fixed ionic strength of 10-3 M NaCl and desired pH conditions by adding microliter quantities of 1 M NaCl and NaOH or 0.01-1 M HCl. These samples were left to reach equilibrium for 24 hs. After that, a given number of microliters of 0.010 M PMG were added to vigorously stirred preequilibrated suspensions and the pH was readjusted until constant values were obtained within a few tenths of a pH unit. pH was measured prior to any analysis.
PMG adsorption was calculated from the difference between the total added ligand and that measured in the supernatant after 24 hs of equilibrium. The suspensions (10.0 g/L) were centrifuged and filtered through a Nucleopore filter of 0.05 to 0.45 µm pore size depending on the material. The amount of PMG present in the supernatant was measured as elementary phosphorous by ICP (inductively coupled plasma) emission spectrometry with a Perkin Elmer Optima 3000XL atomic emission spectrometer with a detection limit lower than 0.1µM. In some experiments PMG was evaluated by ion chromatography [31] using a DIONEX DX-100 instrument with a conductivity detector, a sample injection valve, and a 25 µL sample loop. Two plastic anion columns were coupled in series to serve both as precolumn (DIONEX AG-4) and analytical chromatographic column (DIONEX AS-4). The suppressor was regenerated with 50 mM H2SO4 with a flow rate of 12.5 mL/min. A mixture of NaOH/CO3-2 4mM/9mM was chosen as eluent with a flow rate of 1 mL.min-1. The typical experimental error was lower than 5% for all the experimental results.
Zeta Potential (ZP) measurements were performed with a Zeta Potential Analyzer ZetaPlus from Brokhaven Instruments Corporation at 25ºC and constant ionic strength of 10-3 M KCl. Suspensions of soils loaded with different amounts of PMG were prepared at several pH values and the content of total adsorbed PMG was measured. From these results, those samples of similar PMG adsorption density (ΓPMG) were selected and zeta potential of their particles measured. Finally, comparative curves between soils with and without PMG adsorbed as a function of pH were obtained. The samples were prepared by diluting, with their own supernatant, preequilibrated aliquots of the 10.0 g/L samples of soils giving a concentration of approximately 0.1 g/L pH was adjusted using KOH or HCl. The suspension was dispersed and shaken overnight with a magnetic stirrer. The pH of the final supernatant was measured before and after the ZP measurements were made. The optical unit contains a 35 mW solid state laser, red (660 nm wavelenght). ZP was measuring using a 16 V.cm-1 electric field, 15 mA current and 21 count times.
Results and Discussion
The tendency of glyphosate to form coordination compounds with metal ions is noticeable, besides this glyphosate is capable of forming stable complexes with cations in solution. It has been found that glyphosate forms a 1:1 chelate complex with copper involving the carboxylate, amino and phosphonate group [32]. The stability of its complexes clearly shows the tridentate character of this ligand. Barja and dos Santos Afonso [9] using FTIR-ATR found that in aqueous solution Fe(III) forms a 1:1 Fe(III)/glyphosate complex. Barja et al. [2] also determined that the solid state complexes of glyphosate with cobalt, aluminium and iron(III) consist of a chelate in which the phosphonate and the carboxylic acid groups are coordinated to the metal. In this complex the amino group remains protonated and does not coordinate the metal while the phosphonate moiety always binds the metal ion. The carboxylic acid group of glyphosate only coordinates the Fe(III) in solid state, but not in aqueous solution. Consequently, the coordination of Fe(III) with glyphosate in aqueous medium differs from the results obtained in solid state.
Stability constants of glyphosate-metal complexes have been reported in many publications [5,11,33,34], and the values of the stability constants follow the sequence:
| Fe3+ > Al3+ > Cu2+ > Zn2+ > Fe2+ > Mn2+ > Ca2+ ≈ Mg2+ | (1:1 chelates) |
where 1:1 means 1 glyphosate molecule per cation. More recently the stability constant values were reported in the IUPAC Technical Report 2001 [35].
Glyphosate is immobilized in soils due to the formation of surface complexes with the metal ions [24,25] in a similar way to aqueous solution. In some studies it has been found that the content of iron and/or aluminum oxides is important for the adsorption of glyphosate, and that these soil constituents are the main adsorbents of glyphosate in soil [20,36-38]. Other studies show that the clay content, clay type or CEC (Cation Exchange Capacity) may be the most important soil factors for glyphosate adsorption [14,39]. Normally, isotherms for glyphosate adsorption are of L-type [14,39,40], and have been fitted by Freundlich [14,39,40], the extended Freundlich [41] or the Langmuir isotherm [36,39].
Langmuir adsorption isotherms were determined and their KL and Γmax evaluated (Table 3). The surface recovery and the adsorption isotherm of this herbicide on soil fractions from different provinces of Argentina (Santa Cruz, Misiones, and Corrientes) followed similar patterns to those of the pure minerals that form this soils (Figure 1).
Table 3. Maximum adsorption densities ( Γmax) and Langmuir constants (KL) for PMG adsorbed onto goethite and soils at pH 4 and 7. * Data obtained from Reference [24].


Figure 1. Adsorption isotherms of PMG on soil suspensions at different pH values. Suspensions are 10.0 g of soil/L. Symbols (¨ ) and ( ◊) for Soil A, ( ■) and ( □) for Soil Ch; (▲) and ( ∆) for Soil N. In all the cases full symbols correspond to pH=4 while open symbols refer to pH=7. Solid lines where calculated using a Langmuir model and data from Table 3. Experimental errors are less than 5%.
In this figure curves were calculated using a non linear regression fitting program (Solver, Excel 10) to approximate a Langmuir shape. The Langmuir constant (KL) and maximum coverage (Γmax) of every system are given in Table 3. Γmax values were normalized with the Sw of the soils for a better comparison.
The adsorption of PMG on the soil surfaces as a function of pH is shown in Figure 2. The curve patterns are also similar to those previously reported for anion adsorption on hydrous ferric oxides [42]. Nevertheless there is some disagreement about the effect of pH on glyphosate adsorption on soil. Some studies state that adsorption of glyphosate is not strongly dependent on pH [7,14,43], whereas others find that there is a dependence on pH [16,37,40,41,44,45]. The effect of pH may be due to the influence on the charge of the glyphosate molecule and the surface charge of the adsorbents. The adsorption of glyphosate on soils was markedly affected also by changes in the concentration of the background electrolyte [45], whereas glyphosate adsorption on goethite was only slightly influenced [46].

Figure 2. pH dependence of the PMG adsorption onto Soil A. Suspensions are 10.0 g of solid/L and [PMG]= 4.0 10-3 M
Table 3 shows that the changes in affinity of PMG for the surface (given by the value of ∆KL) for a constant ∆pH are ∆KL/∆pH = -0.065 and -4.66 10-5 for goethite and kaolinite respectively. The affinity changes for the A, Ch and N soils are -2.03 10-4, -4.08 10-4 and -9.0 10-5 respectively. KL values decreases, from pH=4 to pH=7, 87.5 %, 50.0%, 81.3 %, 94.2 % and 53.8% for goethite, kaolinite and soil A, Ch and N respectively. The difference among the decrease percentages of Ch and A and N soils are due to the iron content of these soils. Soil Ch has a different behaviour than goethite, kaolinite and soils A and N due to its dissimilar mineralogical composition. Soil A shows a behavior similar to those of goethite and kaolinite that are the main minerals present in this sample. The different PMG adsorption behaviour of N soil would be assigned to a higher amount of iron incorporated in kaolinite structure (40%) than that obtained for A soil (28%) as determined by Mössbauer Spectroscopy [47].
Generally the amounts of glyphosate adsorbed in soils are small compared with the amounts adsorbed by pure minerals. The adsorption of PMG onto goethite at pH higher than pHiep is not favored due to the negatively charged surface of the oxide. Then, it is possible to expect that at high pH the adsorption of PMG on soils could be due to the presence of another solid phase such as clays. The type of clay may affect glyphosate adsorption. Thus, Dion et al. [39] found that glyphosate adsorption followed the sequence illite > beidellite > kaolinite, and Glass [14] found that it followed the sequence montmorillonite > illite > kaolinite. However, these differences may be due to the different clay types, but may also be due to different surface areas. Gimsing and Borggaard [11, 18], reported that the adsorption by kaolinite was dependent on the surface area, whereas the clay type only had a limited effect on the adsorption, especially when compared with the high adsorption on oxides. In the studies by Dion et al. [39] and Glass [14] the surface areas were not reported. Barja et al. [48] showed that the adsorption sequences at low pH is iron oxides > kaolinite > illite when adsorption is normalized by surface areas. The fact that KL at pH 7 have similar values for A and N soils and kaolinite (Table 3) is indicative that at this pH the adsorption process on this soils occur on the kaolinite mineral phase. In addition, if the surface coverage of soil is calculated using the soil model presented by Taubaso et al. [49] with the thermodynamic parameters obtained from the Langmuir model (Table 3), the surface area (Table 2) and the mineral contents percentage (Table 1), the dashed lines plotted in Figure 3 are obtained. This approximation can represent the adsorption process denoted in the same figure by the solid lines with a good agreement.

Figure 3. Adsorption isotherms of PMG on kaolinite and soil suspensions at pH 7.0. Suspensions are 10.0 g of solid/L (O) Kaolinite, ( ◊) Soil A and ( ∆) Soil N. Solid lines were calculated using Langmuir model and data from Table 3. Dashed line were calculated using model from reference [48] and the experimental values from kaolinite isotherm. Experimental errors are less than 5%
With respect to Γmax, the highest values for PMG adsorption are obtained at lower pH and its value decreases with the increase of pH. This trend in the values of Γmax was also observed in the PMG/goethite system [24]. Barja and dos Santos Afonso also found that the values of Γmax for the adsorption of PMG, AMPA (aminomethylphosphonic acid) and MPA (methylphosphonic acid) onto goethite are comparable at similar pH values indicating that the number of surface sites of goethite that are being occupied by the PMG, AMPA and MPA are the same [24]. In this case the sorption materials have a different mineralogical characteristic and the difference in the values of Γmax would be due to the dissimilarity on the number and the identity of surface sites for each solid that are being occupied by the PMG.
The values of Γmax for A and N soils are very close to those estimated from kaolinite using the same approximation than before. In view of these results, it is reasonable to think that the adsorption of PMG to the surface of soils take place in the similar way than to kaolinite surface.
The zeta potential curves for the soil particles with and without adsorbed PMG as a function of pH are shown in Figure 4. This figure shows how the pHiep shifts to lower pH values due to the amount of adsorbed PMG. Similar patterns were also found for PMG adsorbed onto goethite [24], iron oxides adsorbed on soils [50] and removal of organic matter and deferration of tropical soils [51].
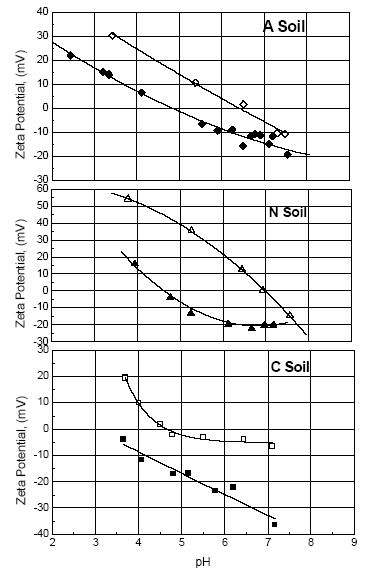
Figure 4. Zeta Potential curves as a function of pH for soils with (solid symbols) and without (open symbols) PMG adsorbed. Experimental conditions are [PMG]=10-2 M, T=25°C and I = 10-3 M KCl. Experimental errors are less than 5%
For goethite the magnitude of the shift in the pHiep to lower values increases with increasing amounts of adsorbed PMG indicating that the PMG adsorbs to the surface of the oxide as an anionic species [24]. Likewise to goethite, the change of the surface charge of the soil samples are negative and increases as the amount of adsorbed PMG increases showing that the surface complexes are more negative than those formed during surface protonation. Thus, PMG surface complexes contain a higher charge density than the protonated or deprotonated surface complexes formed during surface/water interaction. These results provide conclusive evidence about the formation of inner-sphere surface complexes between PMG and the surfaces of goethite or soil particles.
The main mechanism of ligand adsorption is ligand exchange; the surface hydroxyl is exchanged by another ligand [46]. The extent of surface coordination and its pH dependence can be explained by considering the affinity of the surface sites for ligands and the pH dependence of the activity of surface sites and ligands. Since the adsorption of anions is coupled with a release of OH- ions, adsorption is favored at lower pH values. Also, bidentate ligands surface chelates could be formed [46] such as those suggested for the PMG adsorption onto goethite [24]. In this case, two predominating complexes have been identified where the phosphonate group bonds monodentately or bridging bidentately to the surface of iron oxide in an inner sphere mode, while the carboxylate and amino group are noncoordinated to the surface.
Thus, the adsorption process is characterized by the following reactions:
| R≡SOH + -P(O)(OH)-O- + H+ ↔ ≡SO-P(O)(OH)-R + H2O | Ks1 |
| ≡SOH + R-P(O)(OH)-O-↔ ≡SO-P(O)2-R- + H2O | Ks2 |
| 2 ≡SOH + R-P(O)(OH)-O- + H+ ↔ (≡SO)2-P(O)-R + 2 H2O | Ks3 |
Where ≡SOH denotes a feasible surface site for chemical reaction and R means the OOCCH2(NH2)+CH2 group. The stability constant values of PMG/goethite surface complexes logKs1, logKs2 and logKs3 are 10.5, 4.5 and 16.5 respectively [24].
The suitable surface sites for coordination on clays or soils may have a different identity from those on iron oxides surfaces. Moreover, under these conditions the coordination of the soil surfaces may not necessarily form the same surface complexes as those found on iron oxides. In this sense, in the swelling clays it was found that PMG is bound to the external surface sites in a similar way to iron oxides but it is also bound through the positive charged amino group to the surface interlayer sites (unpublished results). To confirm this hypothesis more work should be done.
Conclusions
PMG forms surface complexes on kaolinite and soils with similar maxima surface coverage and the extent of the complexation is dependent on the ligand concentration in solution and pH. The extent of PMG adsorption onto iron oxides is higher than that found on soils or clay. The adsorption behavior of PMG on solids in aqueous suspensions was analyzed as a function of pH and surface coverage. The results obtained suggest that the phosphonate moiety of PMG coordinates to the external surface site of solids with similar structures to that of iron oxides. Inner sphere surface complexes have been suggested where the phosphonate group of PMG bonds to the surface with a similar adsorption behavior observed for PMG onto goethite [24]. These results are potentially important to provide a fundamental understanding of the degradability and bioavailability of PMG in soils and natural waters. However, the complexation of PMG with metal ions and its adsorption onto mineral surfaces might affect its degradation, distribution, and bioavailability in soils and groundwater. The study of the properties of these soil and mineral surface complexes is of high importance in order to assess the implications for control of glyphosate contamination. Glyphosate belongs to a unique class of strongly chelating agents and the adsorption process makes herbicide more persistent in soil.
Acknowledgments
Authors thanks Lic. S. Conconi for her collaboration in the determination of the quantitative mineralogical composition of soils. Authors also acknowledge Universidad de Buenos Aires, Secretaría de Ciencia y Técnica and ANPCyT-FONCyT for financial support through UBACyT TW99 and PICT 13-08893 projects respectively.
References
[1] Ullmann´s Encyclopedia of Industrial Chemistry, Wiley & Sons, 2000 [ Links ]
[2] Barja, B.C.; Herszaje, J.; dos Santos Afonso, .M., Polyhedron, 2001, 20, 1821. [ Links ]
[3] Franz, J.; Mao, M.; Siroski, J., Glyphosate: A Unique Global Herbicide, ACS Monograph 189, Washington DC, 1997. [ Links ]
[4] Kononova, S.V.; Nesmeyanova, M.A., Biochem-Moscow 2002, 67, 184 [ Links ]
[5] Madsen, H.E.L.; Christensen, H.H.; Gottliebpetersen, C., Acta Chem Scand Series A-Physical Inorg Chem 1978, 32, 79. [ Links ]
[6] Wauchope, D.J., Agric Food Chem 1976, 24, 717. [ Links ]
[7] Sprankle, P.; Meggitt, W.F.; Penner, D., Weed Sci 1975, 23, 229. [ Links ]
[8] Subramaniam, V.; Hoggard, P.E., J Agric Food Chem 1988, 36, 1326. [ Links ]
[9] Barja, B.C.; dos Santos Afonso, M., Environ Sci Techn 1998, 32, 3331. [ Links ]
[10] Appleton, T.G.; Hall, J.R.; Mcmahon, I.J., Inorg Chem 1986, 25, 726. [ Links ]
[11] Motekaitis, R.J.; Martell, A.E., J Coord Chem 1985, 14, 139. [ Links ]
[12] Hance, R., J. Pestic. Sci., 1976, 7, 363. [ Links ]
[13] Shoval, S.;. Yariv, S., Clays Clay Miner., 1979, 27, 19. [ Links ]
[14] Glass, R.L., J. Agric. Food. Chem,. 1987, 35, 497. [ Links ]
[15] Mc Connell, J.S.; Hossner, L.R., J. Agric. Food Chem., 1989, 37, 555. [ Links ]
[16] Morillo, E.; Undabeytia, T.; Maqueda, C., Environ. Sci. Technol., 1997, 31, 3588. [ Links ]
[17] Nowack, B.; Stone, A.T., Journal of Colloid and Interface Science, 1999, 214, 20. [ Links ]
[18] Gimsing, A.L.; Borggaard, O.K., Int. J. Environ. Anal. Chem., 2002, 8-9, 545. [ Links ]
[19] Gimsing, A.L.; Borggaard, O.K., Clay Minerals, 2002, 37, 509. [ Links ]
[20] Morillo, E.; Undabeytia, T.; Maqueda, C.; Ramos, A., Chemosphere, 2002, 47, 747. [ Links ]
[21] Hill, Jr.,H.H., Journal of Radioanalytical and Nuclear Chemistry, 2001, 249, 390. [ Links ]
[22] Mc Bride, M.; Kung, K., Soil Sci. Soc. Am. J., 1989, 53, 1668. [ Links ]
[23] Sheals, J.; Sjöberg, S.; Persson, P., Environ. Sci. Technol., 2002, 36, 3090. [ Links ]
[24] Barja, B.C.; dos Santos Afonso, M., Environ. Sci. Technol. 2005, 39, 585 [ Links ]
[25] Gimsing, A L.; dos Santos Afonso, M., Glyphosate in VanBriesen, J.M.; Nowack, B., (Eds) Biogeochemistry of Chelating Agents, ACS Symposium Series Volume 910, 2005 [ Links ]
[26] Atkinson, R.J.; Posner, A.M., Quirk, J.P., J. Inorg. Nucl. Chem., 1968, 30, 2371. [ Links ]
[27] Rietveld, H.M., J. Appl. Crystallogr. 1969, 2, 65. [ Links ]
[28] Rodríguez-Caravajal, J.; Fullprof, a program for Rietveld Refinement and Pattern Matching Analysis. Abstracts XV of Congress of the IUCr, Toulouse, France, pp. 127, 1990 [ Links ]
[29] Lamas, M.C.;.Torres Sánchez, R.M., Geoderma, 1998, 85, 371. [ Links ]
[30] Torres Sánchez, R.M.; Falasca, S., Z. Pfl. Bodenkunde, 1997,160, 223. [ Links ]
[31] Zhu, Y.; Zhang, F.; Tong, C.; Liu, W., Journal of Chromatography A, 1999, 850, 297 [ Links ]
[32] Sheals, J.;. Persson, P.;. Hedman, B., Inorg Chem 2001, 40, 4302 [ Links ]
[33] Smith, P.H.; Raymond, K.N., Inorg Chem 1988, 27, 1056. [ Links ]
[34] Dhansay, M.A.; Linder, P.W., J Coord Chem 1993, 28, 133. [ Links ]
[35] Popov, K.; Ronkkomaki, H.; Lajunen, L.H.J., Pure Appl Chem 2001, 73, 1641. [ Links ]
[36] Piccolo, A.; Celano, G.; Arienzo, M.; Mirabella, A., J Environ Sci Health Part B - Pestic Food Contam Agric Wastes 1994, 29, 1105. [ Links ]
[37] Gimsing, A.L.; Borggaard, O.K.; Bang, M., Europ J Soil Sci 2004, 55, 183. [ Links ]
[38] Gerritse, R.G.; Beltran, J.; Hernandez, F., Aust J Soil Res 1996, 34, 599. [ Links ]
[39] Dion, H.M.; Harsh, J.B.; Hill, H.H., J Radioanal Nuclear Chem 2001, 249, 385. [ Links ]
[40] Mcconnell, J.S.; Hossner, L.R., J Agric Food Chem 1985, 33, 1075. [ Links ]
[41] de Jonge, H.; de Jonge, L.W.; Jacobsen, O.H.; Yamaguchi, T.; Moldrup, P. Soil Sci 2001, 166, 230 [ Links ]
[42] Stumm, W., Chemistry of the Solid-Water Interface, Wiley & Sons, 1992, New York. [ Links ]
[43] Cheah, U.B.; Kirkwood, R.C.; Lum, K.Y., Pestic Sci 1997, 50, 53. [ Links ]
[44] Nicholls, P.H.; Evans, A.A., Pestic Sci 1991, 33, 331. [ Links ]
[45] de Jonge, H.; de Jonge, L.W., Chemosphere 1999, 39, 753. [ Links ]
[46] Gimsing, A.L.; Borggaard, O.K., Clays Clay Min 2001, 49, 270 [ Links ]
[47] Torres Sánchez, R.M.; Okumura, M.; Mercader, R.M., Austr. J. Soil. Res., 2001, 39,423 [ Links ]
[48] dos Santos Afonso, M.; Barja, B.B., Pessagno, R.C.; Tevez, H.R., Biogeochemistry of Chelating Agents Symposium. Extended Abstracts of 226th ACS National Meeting. New York, USA, 2003 [ Links ]
[49] Taubaso, C.; dos Santos Afonso, M.; Torres Sánchez, R.M., Geoderma 2004, 121, 123 [ Links ]
[50] Escudey, M.; Galindo, G., J. Coll. Interf. Sci.,1983, 93, 78 [ Links ]
[51] Osei, B.A.; Singh, B., Geoderma, 1999, 93, 325 [ Links ]














