Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Anales de la Asociación Química Argentina
versión impresa ISSN 0365-0375
An. Asoc. Quím. Argent. v.93 n.4-6 Buenos Aires ene./dic. 2005
REGULAR PAPERS
Two isostructural complexes of Co(II) and Zn(II) with lapacholate, dimethylformamide and water, [M(Lap)2(DMF)(H2O)]
Martínez1, M. A.; Jiménez1, M. C. L. de; Castellano2, E. E.; Piro3, O. E.; Aymonino4, P. J.
1Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, Ayacucho 491, 4000 Tucumán, Argentina
2Instituto de Física de São Carlos, Universidade de São Paulo C.P.369, 13560 São Carlos (SP), Brazil.
3IFLP-LANADI (CONICET), Departamento de Física, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, C.C. 67, 1900 La Plata. Argentina.
4CEQUINOR and LANEFO (UNLP-CONICET), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, C.C. 962, 1900- La Plata, Argentina Fax: +54 221 424 0172/425 9485, E-mail: aymonino@dalton.quimica.unlp.edu.ar
Received July 28th, 2005. In final form October 17th, 2005
Dedicated to the memory of the late Prof. Hans J. Schumacher on the occasion of his 100th birthday
Abstract
The complexes of the title were obtained from lapachol, 2-hydroxy-3-(3-methyl-2- butenyl)-1,4-naphtoquinone, C15H14O3,(LapH), dissolved in dimethylformamide (DMF), and a suspension of cobalt(II) acetate tetrahydrate in 96º ethanol, in one case, and a water solution of zinc acetate dihydrate, in the other. The complexes formed separated from the mother liquors as red-brown, crystalline solids, which were adequately washed and dried. Both complexes, of common formula: [M(Lap)2(DMF)(H2O)], are isostructural, belonging to the triclinic, ![]() (No. 2) space group, with cell constants a = 10.4859(3)Å, b= 12.7319(3) Å, c = 12.8211(3) Å, α = 119.656(2)º, β = 94.480º, γ = 94.181(2)º , Z = 2, for the cobalt complex and a = 10.529(3) Å, b = 12.767(3) Å, c = 12.964(3) Å, α = 119.73(3)º, β = 92.47(3)º, γ = 93.87(3)º, Z = 2, for the zinc complex. Some chemical and spectroscopic properties are also reported.
(No. 2) space group, with cell constants a = 10.4859(3)Å, b= 12.7319(3) Å, c = 12.8211(3) Å, α = 119.656(2)º, β = 94.480º, γ = 94.181(2)º , Z = 2, for the cobalt complex and a = 10.529(3) Å, b = 12.767(3) Å, c = 12.964(3) Å, α = 119.73(3)º, β = 92.47(3)º, γ = 93.87(3)º, Z = 2, for the zinc complex. Some chemical and spectroscopic properties are also reported.
Resumen
Los complejos del título fueron obtenidos a partir de lapachol, 2-hidroxi-3-(3-metil-2- butenil)-1,4-naftoquinona, C15H14O3, (LapH), disuelto en dimetilformamida (DMF) y una suspensión de acetato de cobalto(II) tetrahidrato en etanol de 96º, en un caso y una solución acuosa de acetato de zinc dihidrato, en el otro. Los complejos formados se separaron de los líquidos madre como sólidos cristalinos, castaño-rojizos, que fueron lavados y secados adecuadamente. Ambos complejos, de fórmula común: [M(Lap)2(DMF)(H2O)], son isoestructurales y pertenecen al sistema triclínico, grupo espacial ![]() (No. 2), con constantes de celda: a = 10,4859(3), b = 12,7319(3) , c = 12,8211(3) Å , α = 119,656(2) Å, β = 94,480,γ = 94,181(2)º y Z = 2, para el complejo de cobalto y a = 10,529(3), b = 12,767(3), c =12,964(3) Å, α= 119,73(3), β = 92,47(3), γ = 93,87(3)º, Z = 2, para el complejo de zinc. Se informan también algunas propiedades químicas y espectroscópicas.
(No. 2), con constantes de celda: a = 10,4859(3), b = 12,7319(3) , c = 12,8211(3) Å , α = 119,656(2) Å, β = 94,480,γ = 94,181(2)º y Z = 2, para el complejo de cobalto y a = 10,529(3), b = 12,767(3), c =12,964(3) Å, α= 119,73(3), β = 92,47(3), γ = 93,87(3)º, Z = 2, para el complejo de zinc. Se informan también algunas propiedades químicas y espectroscópicas.
Introduction
Lapachol, [2-hydroxi-3(3-methyl-2-butenil)-1,4-naphtoquinone], is present in several vegetable species and it is used as extracts, in the so called alternative medicine, for the treatment of various sicknesses although without scientifically proved positive results and with the inconvenient of presenting some negative effects in human beings and laboratory animals [1].
It could be of interest the study of the biological activity of complexes of lapachol with cations also biologically active, such as cobalt(II) and zinc (II), which are precisely the subjects of the present work.
Lapachol, (LapH), in alkaline medium, gives place to the lapacholate anion (Lap-) by deprotonation of the phenolic group, which can act as a chelating bidentate ligand through its quinonic and phenolic oxygen atoms, located in orto positions.
In a previous work, we studied the following lapacholates:[Zn(Lap)2(EtOH)2] [2], [Co(Lap)2(DMF)2] and [Co(Lap)2(H2O)2]·2(DMF) [3]. In this work, we are reporting the syntheses and molecular structures as determined by X-ray diffraction, and some other properties of two new hexacoordinated, isostructural complexes of cobalt(II) and zinc(II) with lapacholate, dimethylformamide (DMF) and water: [Co (Lap)2(DMF)(H2O)] (I) and [Zn(Lap)2(DMF)(H2O)] (II).
Experimental
Both complexes were prepared by reaction, at room temperature, of 0.002 mole (0.485 g) of lapachol dissolved in 50 ml dimethylformamide and 0.001 mole of the metal acetate tetrahydrate (0.256 g in the case of cobalt (complex I) and 0.249 g in the case of zinc (complex II) dissolved, respectively, in 50 ml 96º ethanol and the same volume of water. In both cases dark red-brown, rhombohedric crystals were formed. They were separated by filtration, washed either with benzene or with water, respectively and air-dried (lapachol, extracted with benzene from lapacho wood shavings (trees from Argentine Chaco, Tabebuia Ipé genre) and purified by recrystallization from 96º ethanol. Cations contents were determined as the oxides ( Co3O4 and ZnO, respectively) produced in the oxidative thermal decomposition performed in the TG balance (see below).
Lapacholate was determined as lapachol in both cases by dissolving a known amount of each complex in absolute ethanol, adding the minimum amount of 0.1N H2SO4 needed to liberate the lapachol, bringing the solutions to known volumes with absolute ethanol and measuring the absorbancies at 330 nm, where lapachol presents a maximum which obeys Beer's law in a wide concentration interval (ε330 = 2.8 x 103 cm-1 M-1) [4].
Dimethylformamide, water and also, incidentally, lapacholate contents were estimated from TGA curves.
A Shimadzu ATG-50 thermobalance was used at 5ºC/min heating rates, with flowing oxygen (50 ml/min), between ambient temperature and either 500º C (I) or 1000°C (II)
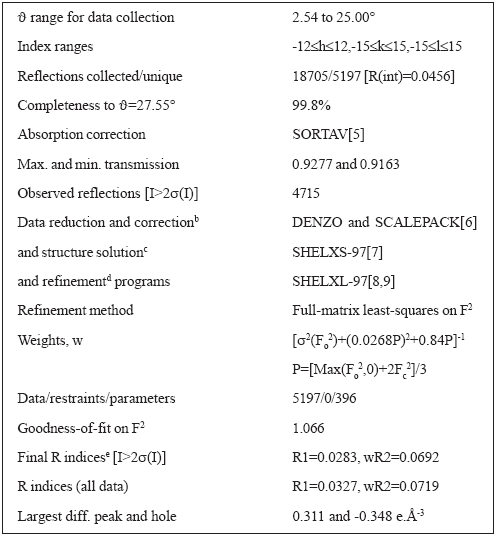
For the structure determinations, single crystals were mounted in a KappaCCD diffractometer. Monochromatic Mo-Kα radiation (graphite monocromator) was used. The structure of the cobalt (II) complex was solved at 120K from 4715 reflections with I>2α(I) and refined by full-matrix least-squares to an agreement R1-factor of 0.0283. Crystal data, data collection procedure, structure determination methods and refinement results are summarized in Table 1.
Table 1. Crystal data and structure solution methods and refinement results for [Co(Lap)2(DMF)(H2O)]


The zinc complex crystals turned out to diffract rather poorly to undertake an X-ray diffraction work precise enough to uncover minor structural differences with the cobalt complex.
IR spectra were run between 4000 and 400 cm-1 with a FTIR Bruker IFS66 spectrophotometer. Samples were included in KBr disks.
Visible spectra of the solids, included in KBr discs, were performed from 400 to 700 nm with a Hewlett Packard 4853 spectrophotometer.
Results
Yields of different preparations were about 65% for I and 70% for II (calculated from the amounts of lapachol used).
The dark red-brown complexes are stable in air, insoluble in water and show unexpectedly different solubilities in benzene (at room temperature: 2.76.10-2 g/l (I); 5.71 g/l (II)).
Results of chemical analyses are the following: Calc. for C33H35O8Co (%): Co, 9.3; dimethylformamide, 11.5; lapacholate, 76.3; water, 2.9. Found: Co, 9.2; dimethylformamide, 10.5; lapacholate, 75.9 (chemical and TG analyses); lapacholate plus water, 80.2 (TGA) (calcd.: 79.1). Calc. for C33H35O8Zn (%): Zn, 10.2; lapacholate, 75.5; dimethylformamide, 11.4; water, 2.8. Found: Zn, 10.2; lapacholate, 75.9 (chemical analysis and TGA); dimethylformamide, 11.1; water, 3.2.
Both complexes belong to the triclinic system, ![]() (No. 2) space group, with cell constants a = 10.4859(3)Å, b= 12.7319(3) Å, c = 12.8211(3) Å, α = 119.656(2)º, β = 94.480º, γ = 94.181(2)º , Z = 2, for I and a = 10.529(3) Å, b = 12.767(3) Å, c = 12.964(3) Å, α = 119.73(3)º, β = 92.47(3)º, γ = 93.87(3)º, Z = 2, for II. For I, intramolecular bond distances and angles around the nuclei are given in Table 2. Tables containing fractional coordinates and equivalent isotropic displacement parameters, listings of full interatomic bond distances and angles, anisotropic thermal parameters for the non-H atoms, and hydrogen atoms positions and isotropic displacement parameters, are included in the Suplementary Material. Figure 1 is an ORTEP [5] drawing of the complex.
(No. 2) space group, with cell constants a = 10.4859(3)Å, b= 12.7319(3) Å, c = 12.8211(3) Å, α = 119.656(2)º, β = 94.480º, γ = 94.181(2)º , Z = 2, for I and a = 10.529(3) Å, b = 12.767(3) Å, c = 12.964(3) Å, α = 119.73(3)º, β = 92.47(3)º, γ = 93.87(3)º, Z = 2, for II. For I, intramolecular bond distances and angles around the nuclei are given in Table 2. Tables containing fractional coordinates and equivalent isotropic displacement parameters, listings of full interatomic bond distances and angles, anisotropic thermal parameters for the non-H atoms, and hydrogen atoms positions and isotropic displacement parameters, are included in the Suplementary Material. Figure 1 is an ORTEP [5] drawing of the complex.
Table 2. Interatomic bond distances and angles around cobalt in [Co(Lap)2(DMF)(H2O)].
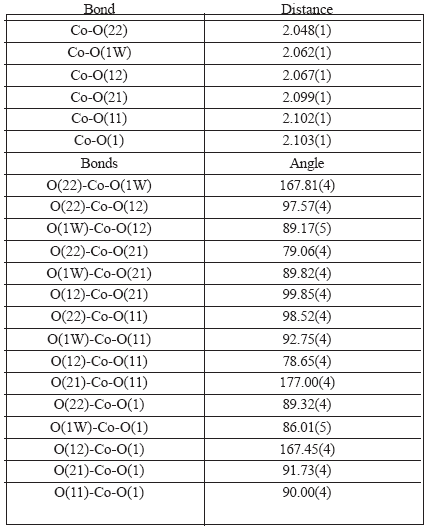
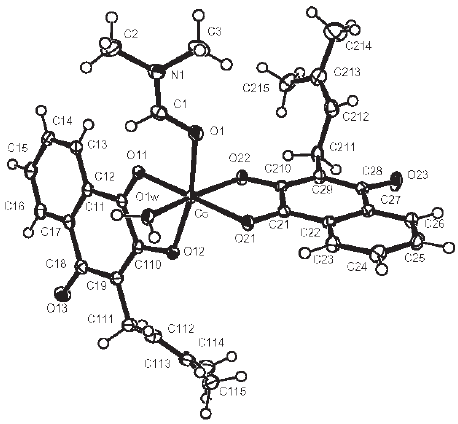
Figure 1. Molecular structure of [Co(Lap)2(DMF)(H2O)]
The cobalt (II) ion is in a distorted octahedral environment, coordinated by two nearly orthogonal (cis) lapacholate anions acting as bidentate ligands through their adjacent carbonyl and phenolic oxygen atoms [Co-O bond distances of 2.102(1) and 2.067(1) Å for ligand 1 and 2.099(1) and 2.048(1) Å for ligand 2, respectively]. The octahedral environment is completed by the oxygen atoms of a dimethylformamide molecule [d(Co-O)=2.103(1) Å] and of a water molecule [d(Co-Ow)=2.062(1) Å] which define with the Co(II) ion a plane nearly perpendicular to the lapacholate planes. The quinonic oxygen atoms are in trans relative positions and the phenolic oxygen's, cis between them but trans with respect to the water oxygen, in one case and to the dimethylformamide oxygen, in the other. This relative configuration of the lapacholate anions is similar to that described for the complex [Cu(Lap)2bpy] [10] but different from the configuration they assume in the complexes previously studied by us [2, 3], were they are disposed at trans positions with respect to each other. Trans O-Co-O angles in the CoO6 core are in the range from 167.45(4) to 177.00(4)° and cis O-Co-O angles are within the 79.06(4) to 98.52(4)° interval. Both lapacholate ligands are nearly planar (rms deviation of fitted atoms from the leastsquares plane is 0.102 Å for lapacholate 1 and 0.058 Å for lapacholate 2). The Co(II) ion departs from these planes 0.073(1) and 0.108(1) Å, respectively. These coordination planes subtend a dihedral angle of 75.18(2)°. The third coordination plane defined by the DMF and water oxygen atoms and the metal makes 84.75(3) and 85.94(3)° angles with the lapacholate coordination planes. The crystal is further stabilized by a pair of medium strength intermolecular O-H...O bonds involving, as donor, the water molecule and as acceptors, the coordinated-tometal phenolic oxygen atom (O12) of the lapacholate 1 ligand of a neighboring, inversion related, complex [d(H2W...O12")=1.968 Å, ∠(O1W-H2W...O12")=175.5°] and the uncoordinated carbonyl oxygen atom (O23) of a lapacholate 2 anion belonging to a neighboring complex displaced by an unit cell translation along the a-axis [d(H1W...O23')=1.814 Å, ∠(O1W-H1W...O23')= 165.4°].
TGA results for I and II are presented, graphically, in Figures 2 and 3, respectively, and numerically, together with calculated results, are in Tables 3 and 4, respectively. In the case of I, Figure 2 and Table 3, it seems that DMF is evolved first, although in two steps, (total weight loss found: 10.2 %, calculated: 11.6 %) and then, up to 500°C, in non differentiated processes, the water molecule and the two Lap- ligands are lost (found: 77.10%, calculated: 75.7 %). Black Co3O4 is left as residue (total weight loss, found and calculated: 87.3%). The curious behaviour of the TG curve beginning at ca. 303 ºC and up to ca. 367 ºC is not an artifact and could be due to the incorporation of oxygen in the solid residue. In the decomposition of II, Figure 3 and Table 4, water leaves first (found: 3.5 %, calc.: 2.8 %), followed by DMF (found: 10.7 %, calc.: 11.4 %) and finally the two Lap- are lost (found and calculated: 73.2 %). If the total weight loss produced between 35ºC and 209ºC is considered, there is a total coincidence between found and calculated weight losses (14.2 %) for the evolution of H2O plus DMF. Weight of residual ZnO is practically coincident with the expected value (found: 12.6 %; calc.: 12.7 %).
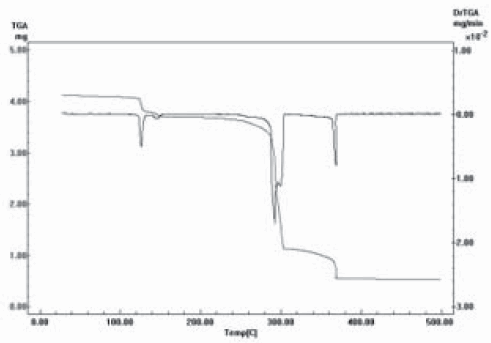
Figure 2. TGA of [Co(Lap)2(DMF)(H2O)]

Figure 3. TGA of [Zn(Lap)2(DMF)(H2O)].
Table 3. TGA of [Co(Lap)2(DMF)(H2O)]
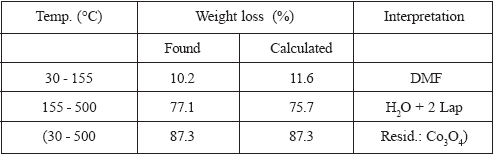
Table 4. TGA of [Zn(Lap)2(DMF)(H2O)]

As the IR spectra of both compounds are nearly identical, only the spectrum of I is shown in Figure 4. Water gives rise to medium strong to strong broad bands centred around 3200 (I) and 3270 (II) cm-1 due to the hydrogen bonded OH stretchings; bands due to the angular deformation seem to be superposed with bands due to the CO groups. Between 2800 and 3000 cm-1 appear bands due to the CH stretching of the CH, CH2 and CH3 groups. Bands due to the stretching of quinonic CO groups coordinated to the nuclei of the complexes, C11O11 and C21O21, are located at 1628 cm-1 and 1632 cm-1 in the spectra of I and II, respectively, and those of the non-coordinated but hydrogen-bonded groups C18O13 and C28O23, appear at 1587 and 1588 cm-1, respectively. These bands are shifted towards FIR (smaller wave numbers) with respect to the corresponding bands of lapachol [4] (1662 and 1641 cm-1) due to the coordination of the C11O11 and C21O21 groups to the nuclei (shifts from 1662 cm-1 to 1628 and 1632 cm-1, respectively) and to the resonance between the para- and orto-naftoquinone forms that involves those carbonyl groups and the non-coordinated, but hydrogen-bonded C18O13 and C28O23 groups (shifts from 1641 to 1587 and 1588 cm-1, respectively). Bands at 1662 cm-1 and 1666 cm-1, in the spectra of I and II, respectively, are assigned to the DMF carbonyl. Those located at 1535 cm-1, in both spectra, should correspond to a stretching mode of the quinonic rings. For the assignment of phenolic C110O12 and C210O22 stretching and other vibrations of the M(Lap)2 groups see [2], where assignments are supported by comparison with spectra of lapachol, sodium, iron(II) and nickel lapacholates, lawsone (as such and as the iron(II) lawsonate) and other 1,4-naphtoquinones.

Figure 4. IR spectrum of [Co(Lap)2(DMF)(H2O)]
The visible spectra of solids I and II, Figure 5, show strong and broad bands with absorption maxima at 530 nm and 497 nm , respectively, that justifies their intense red-brown colours. These bands could be due to the mesomerism that could work in the conjugated system: O12- C110=C12-C18=O13 ↔ O12=C110-C12=C18-O13 (p-quinone ↔ o-quinone) (see [2]).

Figure 5. Visible spectra of solid [Co(Lap)2(DMF)(H2O)] (a) and [Zn(Lap)2(DMF)(H2O)] (b)
Conclusions
In the preparation of complex II, {[Zn(Lap)2(DMF)(H2O)]}, an aqueous solution of Zn(II) acetate dihydrate together with a solution of lapachol in dimethylformamide was used, a fact that explains the presence of H2O and DMF as ligands in the complex but in the preparation of [Co(Lap)2(DMF)(H2O)] (I), as water was not used as solvent, it seems that for its incorporation as ligand the hydration water of cobalt acetate (tetrahydrate) is enough, plus that of 96º ethanol and the humidity present in DMF, which was used without previous dehydration.
The molecular structure, common to both complexes, is characterised by the cis arrangement of the lapacholate ligands, as found in the complex [Cu(Lap)2bpy] prepared by Oliveira et al. [10] but different from that of the complexes already studied by us, i. e.: [Zn(Lap)2(EtOH)2] [2], [Co(Lap)2(DMF)2] and [Co(Lap)2(H2O)2] [3], where the two lapacholate anions are in trans positions.
According to the temperatures of DMF and H2O losses, when the complexes are heated under flowing oxygen, the first of these ligands, in I, seems to be more strongly coordinated, in this case to the cobalt nucleus, than the second; however, in II, it seems to occur just the opposite. Lapacholate is, in both complexes, the ligand more strongly bonded to the metal ion, due to its negative charge and its bidentated condition.
The spectra of solid samples in the visible present, between 450 and 650 nm, broad and strong bands centered at 530 nm (I) and 497 nm (II) that explain the intense red-brown colour of the substances, which could be attributed to the mesomerism that might occur in the conjugated system: O12-C110=C12-C18=O13 ↔ O12=C110-C12=C18-O13 (p-quinone ↔ oquinone) [2].
Suplementary material
Crystallographic data have been deposited with the Cambridge Crystallographic Data Center under deposition number 254747 for complex I. Copies of the information may be obtained, free of charge, from The Director, CCDC, 12 Union Road, Cambridge, CB2, IEZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
Acknowledgements
To Professor M. Poch, Cátedra de Farmacognosia, Facultad de Bioquímica, Química y Farmacia, UNT, for providing us with lapachol. To CIUNT and UNT (M.A.M., M.C.L.), FAPESP and CNPq (E.E.C., O.E.P.) and CONICET and UNLP (O.E.P., P.J.A.) for financial support.
References
[1] Sparreboom, M..C.; Cox, M.C.; Acharya, M.R.; Figg, W.D. J. Clin. Oncol. 2004, 22(12), 2489. [ Links ]
[2] Martínez, M.A.; Jiménez, C.L. de; Castellano, E.E.; Piro, O.E.; Aymonino, P.J., Coord..Chem. 2003, 56, 803. [ Links ]
[3] Martínez, M.A.; Jiménez, C.L. de; Castellano, E.E.; Piro, O.E., Aymonino, P.J., XIII Meeting of the Argentine Physical and Inorganic Chemistry Society, Bahía Blanca, Argentina, April 7-10, 2003. [ Links ]
[4] Da Guia Mello Portugal, S.; Machuca Herrero, J.O.; Mark Brinn, E., Bull. Chem. Soc. Jpn.. 1997, 70, 2071. [ Links ]
[5] Blessing, R.H., Acta Cryst. 1995, A51, 33. [ Links ]
[6] Otwinowski, Z.; W. Minor, in: Methods in Enzymology, Carter Jr. C.W.; Sweet, R.M., Eds., Academic Press, New York, 1997, 276, 307-326. [ Links ]
[7] Sheldrick, G.M., SHELXS-97. Program for Crystal Structure Resolution, University of Göttingen, Göttingen, Germany, 1997. [ Links ]
[8] Sheldrick, G.M., SHELXL-97. Program for Crystal Structures Analysis, University of Göttingen, Göttingen, Germany, 1997. [ Links ]
[9] Johnson, C.K. ORTEP-II. A Fortran Thermal-Ellipsoid Plot Program. Report ORNL 5138,. Oak Ridge National Laboratory, Tennessee, USA, 1976. [ Links ]
[10] De Oliveira, E.H.; Medeiros, G.E.A.; Pepe, C; Brown, M.A.; Tuck., D. G., Can. J. Chem. 1997, 75, 499. [ Links ]














