Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Anales de la Asociación Química Argentina
versión impresa ISSN 0365-0375
An. Asoc. Quím. Argent. v.93 n.4-6 Buenos Aires ene./dic. 2005
REGULAR PAPERS
Preparation, Spectroscopic And Thermal Studies Of The Bridged Isocyanato Complex [Ni2(NCO)2(H2O)4]X2, (X= ½SO4 2− Or NO3− )
Gaballa, A.S.1 ; Teleb, S.M.2; Nour, E.M.2
1Faculty of Specific Education, Zagazig University, Zagazig, Egypt
2Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt Fax: +20 55 2345452, E-mail: akmalsg@yahoo.com
Received August 2nd, 2005. In final form October 10th, 2005
Abstract
The preparation of solid complexes [Ni2(NCO)2(H2O)4]SO4 and [Ni2(NCO)2(H2O)4]- (NO3)2 during the reaction of biuret with NiSO4 ×7H2O and Ni(NO3)2 ×6H2O, respectively, in aqueous solutions at elevated temperature are described. The reaction products are characterized through elemental analyses and infrared measurements. The IR spectra of the new complexes clearly indicate the absence of bands due to coordinated biuret, but instead show characteristic bands of bridged isocyanato ligands and coordinated water. TG and DTA thermograms of the complex [Ni2(NCO)2(H2O)4]SO4, as well as the IR spectrum of its final thermal decomposition product were recorded. The data obtained agree quite well with the expected structure. Detailed mechanisms for complex formation and their mode of thermal decomposition are given.
Resumen
En este trabajo se describe la preparación, en solución acuosa y a elevadas temperaturas, de los complejos sólidos [Ni2(NCO)2(H2O)4]SO4 y [Ni2(NCO)2(H2O)4]-(NO3)2 durante la reacción de biuret con NiSO4 ×7H2O y Ni(NO3)2 ×6H2O, respetivamente. Los productos de reacción fueron caracterizados mediante análisis elemental y espectroscopía IR. Los espectros IR muestran la ausencia de bandas correspondientes a biuret coordinado. En cambio, se observan bandas correspondientes a ligandos isociano puentes y coordinados al agua. Se registraron termogramas TG y DTA del complejo [Ni2(NCO)2(H2O)4]SO4, así como del producto de su descomposición. Los información obtenida concuerda muy bien con la estructura esperada. Se describen mecanismos de formación de los complejos y de sus descomposiciones térmicas.
Introduction
The biuret molecule is known to act as a good donor ligand. It has the ability to from stable complexes with metal ions [1-4]. The formation of oxygen to metal bonds between biuret molecules and metal ions occurs in acidic and neutral media [4,5]. However, in alkaline medium, biuret molecule coordinates to metal ions through amide nitrogen atoms [4-8]. The chelation mode of biuret molecule in many biuret-metal solid complexes have been estimated by infrared spectral measurements and established by X-ray analysis [4,7].
In the last few years, this research group is engaged in studying the role of metal ion in the mode of decomposition of coordinated urea in many metal-urea complexes at elevated temperature and very interesting results were obtained [9-14]. It was found that the formed reaction products depend upon the nature of the metal ions used as well as on the nature of the counter ions associated with the metal ions. To continue our investigation in this area, we report here, the study of the related nickel(II)−biuret complexes at high temperature as biuret molecule is looked as a condensed form of two urea molecules. The nature of the obtained reaction products were identified through their elemental analyses, infrared spectra as well as thermal analysis. The obtained results enabled us to make an assessment of the nature of the decomposition of Ni(II)−biuret complexes beside the understanding of the bonding and structures inherent in reaction products.
Experimental
Biuret (Merck) was purified by using recrystallization from ethyl alcohol. NiSO4.7H2O and Ni(NO3)2.6H2O were of Analar grade.
The parent complexes [Ni(biuret)2]X2 (where X= ½SO4 2− or NO3 −) were prepared by a method similar to that described for the preparation of the related complex [Ni(biuret)2]Cl2 [4]. A hot solution of the Ni(II) salt (10 mmol) in absolute ethanol (80 mL) was added to a hot solution of biuret (2.06 g., 20 mmol) in ethanol (160 mL). The mixture was refluxed for about ½ h. The precipitated complex (pale green) was filtered off, washed with ethanol and dried under vacuum over P2O5. Anal. Found (Calcd for [Ni(biuret)2]SO4: C4H10N6NiO8S; 360.93): C, 13.41 (13.31); H, 2.80 (2.79); N; 23.32 (23.28); Ni, 16.30 (16.27); S, 8.86 (8.88).
Anal. Found (Calcd for [Ni(biuret)2](NO3)2: C4H10N8NiO10; 388.88): C, 12.56 (12.35); H, 2.60 (2.59); N, 28.32 (28.81); Ni, 15.50 (15.10).
The binuclear complexes [Ni2(NCO)2(H2O)4]SO4 and [Ni2(NCO)2(H2O)4](NO3)2 were prepared by mixing 0.1 mole of the Ni(II) salt in each case dissolved in 50 mL H2O with 0.2 mole of the biuret dissolved in 50 mL H2O. The reaction mixture was heated on a water bath to ca. 90−95 °C with continuous stirring. A green complex began to precipitate after 25 hours and its amount increases by increasing the heating time. The precipitated products were separated by filtration and washed several times with hot water, then dried in a desiccator under vacuum over P2O5. However, the same products [Ni2(NCO)2(H2O)4]SO4 or [Ni2(NCO)2(H2O)4](NO3)2 could also be obtained by dissolving the corresponding parent complex [Ni(biuret)2]SO4 or [Ni(biuret)2](NO3)2, respectively in 100 mL distilled water and heated on a water bath at ca. 90- 95 °C with constant stirring. Anal. Found (Calcd for [Ni2(NCO)2(H2O)4]SO4: C2H8N2Ni2O10S; 369.58): C, 6.42 (6.50); H, 2.30 (2.18); N, 7.60 (7.58); Ni, 32.00 (31.77); S, 8.48 (8.68). Anal. Found (Calcd for [Ni2(NCO)2(H2O)4](NO3)2: C2H8N4Ni2O12; 397.53): C, 6.00 (6.04); H, 2.30 (2.03); N, 14.20 (14.09); Ni, 30.10 (29.54).
The parent complexes, [Ni(biuret)2]X2 and the reaction products [Ni2(NCO)2(H2O)4]X2 (where X = ½SO4 2− and NO3−) were identified by their elemental analyses, infrared spectra as well as thermal analysis (DTA and TG). Nickel(II) content was determined by atomic absorption method using a PYE UNICAM atomic absorption spectrophotometer SP 1900 fitted with a nickel lamp. The infrared spectra of all reactants and the formed complexes were recorded as KBr discs using a Perkin Elmer 1650 ratio recording infrared spectrophotometer. Thermogravimetric (TG) and differential thermal analysis (DTA) were carried out using a Shimadzu TGA.50H and DTA.50 computerized thermal analysis system. The rate of heating of the samples was kept at 10 °C min−1. A 15 mg sample was analysed under N2 flow at 30 ml min−1.
Results and discussion
Two greenish solid complexes [Ni2(NCO)2(H2O)4]SO4 and [Ni2(NCO)2(H2O)4]-(NO3)2 were obtained in aqueous solution at elevated temperature, ca. 95 °C, either by the reaction of biuret with NiSO4 .7H2O or Ni(NO3)2 .6H2O, respectively, or through the decomposition of the corresponding parent Ni(II)−biuret complexes. The infrared spectra of biuret, parent complexes and of the reaction products are shown in Figure 1. Band assignments of these spectra are given in Table 1. The IR spectra of the parent complex ion [Ni(biuret)2]2+, Figure 1, B and C, indicate a coordinated biuret ligand associated with nickel ions. The infrared spectra of the products obtained on reacting Ni(II) ions with biuret at high temperature, Figure 1, D and E, clearly show four features in common. (i) The absence of bands due to coordinated biuret, (ii) The presence of a medium to strong new band observed at about 2185 cm−1 related to the N≡C bond stretching vibration and is characteristic for a bridged isocyanato ligand [10, 15, 16]. (iii) A set of bands lies in the region 3642−3421 cm−1 characteristic of coordinated water [10, 16-18]. (iv) The regions of 700-200 cm−1 in the spectra of the two complexes are very similar in terms of band positions and are related to the various Ni(II)−ligands vibrations (Ni−N) and (Ni−O), Table 1. On the bases of elemental analysis as well as the infrared spectra of these products, we suggest the formation of the binuclear complex [Ni2(NCO)2(H2O)4]X2 (where X = ½SO42− and NO3 −). The same binuclear complex ion [Ni2(NCO)2(H2O)4]2+ has been obtained in a previous investigation on reacting nickel(II) chloride or nitrate with urea at high temperature [10].
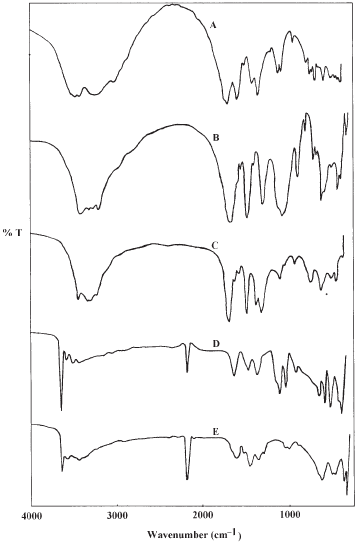
Figure 1. The infrared spectra of (A) Biuret, (B) [Ni(biuret)2]SO4, (C) [Ni(biuret)2](NO3)2, (D) [Ni2(NCO)2(H2O)4]SO4 and (E) [Ni2(NCO)2(H2O)4]((NO3)2.
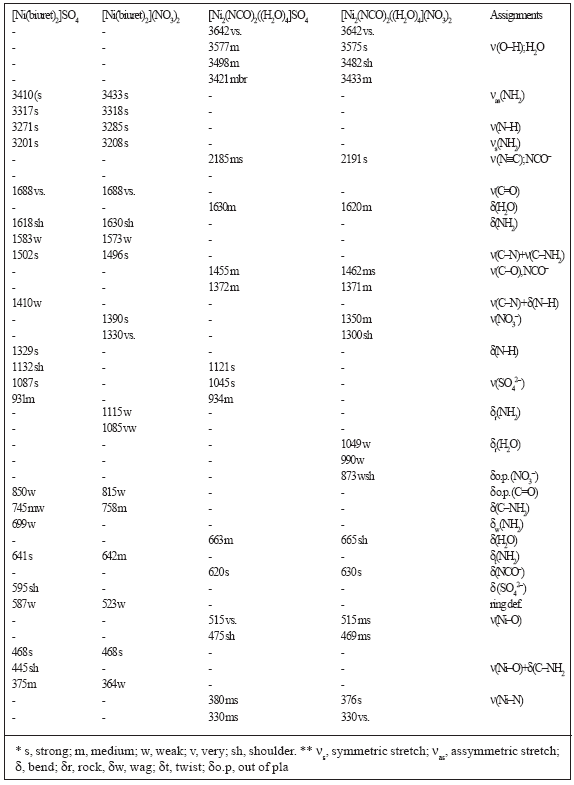
Table 1. Infrared frequencies* (cm −1) and band assignments** for the [Ni(biuret)2]X2 and [Ni2(NCO)2(H2O)4]X2 (X = ½SO4 2 −, NO3−) complexes
It is known that biuret coordinates to nickel(II) ions at lower temperature to form the complex ion [Ni(biuret)2]2+ [4]. The formation of the bridged isocyanato complex, [Ni2(NCO)2(H2O)4]X2 (X = ½SO42− or NO3 −) from the corresponding parent complex at high temperature may be understood as indicated by the following reactions:

The formation of the green solid complexes, [Ni2(NCO)2(H2O)4]SO4 and [Ni2(NCO)2(H2O)4](NO3)2 clearly indicate that Ni(II) ions decompose the coordinated biuret to isocyanate in the same manner as they do with urea at high temperature. However, the interconversion between biuret and isocyanate and also between urea and isocyanate are known in the literature [3].
Complete assignments of the infrared bands associated with the spectra of parent complexes, [Ni(biuret)2]X2, and of the binuclear complexes, [Ni2(NCO)2(H2O)4]X2 (where X = ½SO4 2− and NO3−) are given in Table 1. For the parent complexes, all the assignments lie in the expected regions and agree quite well with the related complexes, [Ni(biuret)2]X2 (where X = Cl−, Br−) [4,5]. For the spectra of the binuclear complexes, [Ni2(NCO)2(H2O)4]SO4 and [Ni2(NCO)2(H2O)4](NO3)2, a group of four bands lies in the region 3642−3421 cm−1 characteristic for the stretching motions of the four coordinated water molecules [10,16-18]. The corresponding bending motion δ(H2O) are observed in the expected region around 1630 cm−1 [16]. On the other hand, the various ν(N≡C) of the bridged isocyanato ligands are observed in the expected region as a strong band at nearly 2185 cm−1. The ν(C−O) of the NCO group is assigned as two bands for each complex around 1455 and 1372 cm−1, while the bending motion of the type δ(NCO) in the two complexes is assigned as a strong band at 620 and 630 cm−1 for the sulphate and nitrate complexes, respectively. The nickel(II)−ligand bond vibrations of the type ν(Ni−O) and ν(Ni−N) are observed at 515 and 380 cm−1, respectively [10,15,20]. The assignments of these bands and the other bands associated with spectra of the complexes (Table 1) agree quite well with those known for other related complexes [10, 16, 19, 20].
To make sure about the structure of these complexes, thermogravimetric (TG) and differential thermal analysis (DTA) were carried out for the solid complex [Ni2(NCO)2(H2O)4]SO4 under N2 flow, and its thermograms are shown in Figure 2. The obtained thermal data strongly support the proposed complex formula and indicate that the mode of thermal decomposition of the complex, [Ni2(NCO)2(H2O)4]SO4 occurs in one stage at a maximum temperature of 308.7°C and is accompanied by a weight loss of 23 % corresponding to the loss of two carbon dioxide molecules, in good agreement with the calculated values of 23.82 % according to the following reactions:
![]()

Figure 2. DTA and TG curves for [Ni2(NCO)2(H2O)4]SO4 complex.
The residue after this stage is expected to be a mixture of NiO and (NH4)2SO4. The infrared spectrum for the residue left after this temperature strongly supports this conclusion, Figure 3. The spectrum shows the characteristic bands associated with (NH4)2SO4.
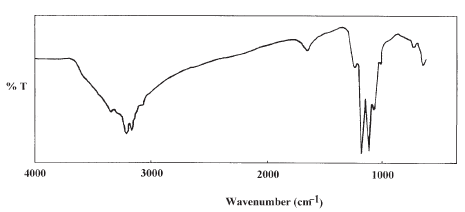
Figure 3. Infrared spectrum of the final thermal decomposition product, ((NH4)2SO4+NiO)
References
[1] Kato, M.; Komuro, U.; Sone, K.; J. Chem. Soc. Japan, 1954, 75, 1134. [ Links ]
[2] Kato, M.; Z. Phys. Chem., 1960, 23, 375. [ Links ]
[3] Kurzer, F.; Chem. Rev., 1956, 56, 95. [ Links ]
[4] Mclellan, A.W.; Melson, G.A.; J. Chem. Soc. A, 1967, 137. [ Links ]
[5] Kedzia, B.B.; Armendarez, P.X.; Nakamoto, K.; J. Inorg. Nucl. Chem., 1968, 30, 849. [ Links ]
[6] Aida, K.; Musya, Y., Kinumaki, S.; Inorg. Chem., 1963, 2, 1268. [ Links ]
[7] Freeman, H.C.; Smith, J.E.W.L.; Taylor, J.C.; Nature, Lond., 1959, 184, 707. [ Links ]
[8] Thamann, T.J.; Loehr, T.M.; Spectrochim. Acta, 1980,36A, 751. [ Links ]
[9] Nour, E.M.; Rady, A.H.; Synth. React. Inorg. Met. Org. Chem., 1991, 21(8), 1153. [ Links ]
[10] Nour, E.M.; Rady, A.H.; Transition Met. Chem., 1991, 16, 400. [ Links ]
[11] Teleb, S.M.; Thermochim. Acta, 1993, 228, 131. [ Links ]
[12] Teleb, S.M.; Sadeek, S.A.; Nour, E.M.; J. Phys. Chem. Solids, 1993, 54, 85. [ Links ]
[13] Nour, E.M.; Al-Thani, M.J.; J. Phys. Chem. Solids, 1989, 50, 183. [ Links ]
[14] Nour, E.M.; Teleb, S.M.; Al-Khsosy, N.A.; Refat, M.S.; Synth. React. Inorg. Met. Org. Chem., 1997, 27(4), 505. [ Links ]
[15] Forster, D.; Goodgame, D.M.L.; J. Chem. Soc., 1965, 262. [ Links ]
[16] Nakamoto, K., "Infrared Spectra of Inorganic and Coordination Compounds" John Wiley, New York, 1978. [ Links ]
[17] Beatie, I.R.; Gilson, T.R.; Ozin, G.A. ; J. Chem. Soc. A, 1969, 534. [ Links ]
[18] Adams, D.M.; Lock, P.J.; J. Chem. Soc. A, 1971, 2801. [ Links ]
[19] Bellamy, L.J., "Infrared Spectra of Complex Molecules" Chapman, Hall. London, 1975. [ Links ]
[20] Ross, S.D., "Inorganic Infrared and Raman Spectra", McGraw Hill, London, 1972. [ Links ]














