Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Anales de la Asociación Química Argentina
versión impresa ISSN 0365-0375
An. Asoc. Quím. Argent. v.93 n.4-6 Buenos Aires ene./dic. 2005
REGULAR PAPERS
Fatty Acids And Sterols In Seeds From Wild Species Of Berberis In Argentine Patagonia
Mazzuca, M.1; Miscoria, S.A.1; Rost, E.2, Balzaretti, V.T.1
1Departments of Chemistry and 2Industry. Universidad Nacional de la Patagonia San Juan Bosco, Km 4, (9000) Comodoro Rivadavia, Chubut, Argentina
Fax: +54 297 455 0339, E-mail: mazzucam@unpata.edu.ar
Received June 2nd, 2005.
In final form December 8th , 2005
Abstract
Seeds of Berberis heterophylla and Berberis buxifolia were analyzed for lipids, fatty acids and sterols contents. The lipids in seeds contained a relatively large proportion of unsaturated fatty acids, with linolenic, linoleic and oleic being predominant. β-sitosterol was the major sterol in both species, followed by stigmasta-5,24(28)-dien-3 β-ol and campesterol. The seeds of B. heterophylla were the richest in total lipid content.
Resumen
Se analizaron los ácidos grasos, lípidos totales y esteroles en semillas de Berberis heterophylla y Berberis buxifolia. Los lípidos presentes en las semillas contenían un porcentaje relativamente alto de ácidos grasos insaturados, predominando el ácido linolénico, linoleico y oleico. El mayor esterol fue β-sitosterol, seguido por estigmasta-5,24(28)-dien-3 β-ol y campesterol. Las semillas de B. heterophylla fueron las más ricas en lípidos totales.
Introduction
Berberis heterophylla Jussieu and Berberis buxifolia Lamarck, commonly named "michay" and "calafate" respectively [1-3], belong to the family Berberidaceae and grow as wild species in many regions of the Patagonian steppes and Subantartic forests [1,4]. Its fruits, which have an agreeable flavor, consist of a pulp with many small seeds which are also edible, and are consumed raw or cooked to make jelly or juice with water and sugar, and alcoholic beverages [1,5]. By fermentation of fruits of B. buxifolia, wine of calafate is obtained [1]. By maceration, strong alcoholic beverages are obtained. It is also used for coloring wines [5].
Fruits of Berberis heterophylla are also cited as one of the edible wild resources most often eaten by the Mapuche community in Patagonia [2], and Berberis species serve as forage for wildlife and domestic herbivores in Patagonia, i.e. ovine, choique, guanaco and hare [6].
At present, calafate marmalade is considered a regional Patagonian product, and can be commercially obtained in many places destined mainly to the tourist market.
There are few studies published on the chemical composition of B. buxifolia seeds [5], and none describes the chemical composition of B. heterophylla seeds. Continuing with our study on native regional vegetation [7,8], and in view of the growing importance of knowing the quality of food, we have analyzed the composition of fatty acids, sterols and other steroids present in the seed of both B. heterophylla and B. buxifolia with the aim of contributing to the knowledge of the nutritive characteristics of these seeds.
Experimental
Chemicals
Acetyl chloride was purchased from Fisher Scientific (Fair Lawn, NJ, USA). Heptadecanoic acid methyl ester (C17), 5-α-cholestane and cholesterol were supplied by Sigma (St. Louis, MO, USA). Fatty acid methyl ester reference standards were purchased from Supelco, Inc. (Bellefonte, PA, USA) and Nu-check-prep, Inc. (Elysian, MN, USA) whereas Generol (sterol mixture: 58 % β-sitosterol; 31% campesterol and 5% stigmasterol) were purchased from General Mills, Inc. (Minneapolis, MN, USA). TLC aluminum sheets coated with silica gel 60 F254 (20 cm x 20 cm x 0.2 mm of film thickness) were supplied from Merck (Darmstadt, Germany).
Samples
Fresh fruits of Berberis heterophylla, origin Rada Tilly city, Chubut, Argentina, and Berberis buxifolia, origin Calafate city, Santa Cruz, Argentina, were collected by our laboratory staff in December 2003 and January 2004, respectively. Samples were identified by Prof. M. E. Arce, Cátedra de Plantas Vasculares, Universidad Nacional de la Patagonia San Juan Bosco. Seeds were separated from pulp, air dried two weeks in the dark, and then homogenized in a coffee bean grinder and passed through a 1mm sieve (Retsch, GmbH, Haan, Germany). Moisture content was made by drying at 50°C for 4 hs. All determinations described below were made in triplicate.
Fatty acid analysis
Ground seed samples (50 mg) with 10 µl C17 internal standard (25 mg diluted in 1 mL toluene) were subjected to direct transesterification with 2 mL of acetyl chloride in methanol (1:20 v/v) in test tubes according to the literature [9]. After the reaction, the tubes were cooled in water at 25°C and the reaction mixture was diluted with 2 mL of water and extracted three times with 2 mL of hexane. The hexane phase (about 6 mL) was dried under a gentle stream of nitrogen at atmospheric pressure and room temperature, and the fatty acid methyl esters were resuspended in 50 µl of hexane for injection into the chromatograph.
GC determination of fatty acids composition was done on a HP 5890 (Hewlett Packard, Avondale, Pennsylvania, USA) GC, equipped with a flame ionization detector (FID), with a 30 m, 0.53 mm ID and 10 µm film thickness, Innowax (Crosslinked Polyethylene Glycol), capillary column (Hewlett Packard, Avondale, Pennsylvania, USA). Helium was used as carrier gas, flow rate of 6 mL /min (50 kPa, measured at 150°C), split 5:1. The temperatures at the injector port and detector were 240°C and 270oC respectively. Column temperature was controlled with a temperature elevation program, which was initially set at 150 °C for 5 min, then increased to 240°C at the rate of 4°C min-1, and held at that temperature for 15 min. Standard fatty acid methyl ester mixtures were run under identical conditions to identify the compounds on the basis of their retention times. Retention times obtained were matched with those of reference compounds. The theoretical iodine number was calculated according to Carreras et al. [10].
Lipid extraction
Ground seeds were extracted according to the modified Folch procedure [11] in order to determine the weight of lipid relative to dry matter. Solvent removal under reduced pressure was performed in a rotavapor model R-114 (Büchi, Flawil, Switzerland), with a water bath model B-480 (Büchi, Flawil, Switzerland).
Sterol analysis
Lipid extracts (45 mg) with 50 µL of 5α-cholestane internal standard (24 mg diluted in 8 mL ethanol), were refluxed with 1 M ethanolic potassium hydroxide (9 mL, 120 min) [11]. The saponification process was monitored by thin layer chromatography developed with hexane/ diethyl ether/acetic acid (90/10/1 v/v/v) as the eluent. The developed TLC plate was sprayed with a solution freshly prepared of ferric chloride (50 mg) in a mixture of water (90 mL), acetic acid (5 mL) and sulphuric acid (5 mL) spray reagent. After heating at 100°C for 3-5 min, the sterol spots are indicated by a red-violet color (Rf 0.3). After the reaction, the mixture was diluted in water (22.5 mL), and extracted three times with 22.5 mL hexane-diethyl ether (1:1). The combined extract was washed with distilled water until neutral to pH paper and dried under a gently stream of nitrogen at atmospheric pressure, room temperature. Removal of solvents yields an unsaponifiable residue. The sterol fraction was separated from the unsaponifiable material by thin layer chromatography, using aluminium sheets coated with 0.2 mm of silicagel 60 F254 (Merck, Darmstadt, Germany), and developed with hexane/diethylether (65/35 v/v) as the eluent. The sterol fraction was resuspended in 100 µL of ethanol for injection into the chromatograph. GC analysis of sterols was performed with a HP 5890 (Hewlett Packard, Avondale, Pennsylvania, USA) GC, equipped with a flame ionization detector (FID), with a 30 m length, 0.25 mm ID and 0.25 µm film thickness, HP-5 (Crosslinked 5% Phenyl Methyl Siloxane) capillary column (Hewlett Packard, Avondale, Pennsylvania, USA). Helium was used as carrier gas, flow rate of 2.2 mL/ min (70 kPa, measured at 250°C), split 20:1. The temperatures at the injector port and detector were 320°C and 360°C, respectively. The oven temperature was controlled with a temperature elevation program, which was initially set at 250°C for 3 min, then increased to 320°C at the rate of 7°C min-1, and held at that temperature for 20 min. Standard sterol mixtures were run under identical conditions to identify the compounds on the basis of their retention times. GC-MS was performed on a VG Trio II (Fisson Instruments / VG Analytical, Manchester, UK) mass spectrometer. Fragmentation patterns and retention times obtained were matched with those of reference compounds.
Results and discussion
The moisture content of Berberis seeds were 3.5 (± 0.2) % and 3.7 (± 0.2) % for B. heterophyla and B. buxifolia, respectively. Total lipids on dry weight basis were 79.9 (± 0.3) mg g-1 (7.9 %) for B. heterophylla and 66 (±0.2) mg g-1 (6.6 %) seeds for B. buxifolia.
The results from fatty acid composition are given in Table 1. Eleven fatty acids were identified. Linolenic acid was the predominant fatty acid in both species followed by linoleic and oleic acid. Palmitic acid was the major saturated fatty acid identified in both species. The percentages of the main fatty acids were similar to those determined by Sztarker et al. by B. buxifolia, but the percentages of the minor fatty acids (20:0; 20:1; 22:0 and 24:0) were considerably higher than those obtained in that previous work, in which they were identified as trace [5]. Seeds from both studied Berberis species are good sources of α-linolenic and linoleic acids. The ratio of polyunsaturated to saturated were 6.4 and 6.1 for B. heterophylla and B. buxifolia respectively. Iodine number were 170 and 176 for B. heterophylla and B. buxifolia respectively. Furthermore, the ratio omega-6/omega-3 are low, less than 1 in both species of Berberis, and this characteristic is consistent with the recommended adequate intakes for adults and infants held in the workshop on the essentiality and recommended dietary intakes for omega-6 and omega-3 fatty acids at the National Institute of Health (NIH) in Bethesda, Maryland, USA, 1999[12]. The α-linolenic acid is known to improve some diseases, such as neuron degeneration, scarly dermatitis [13], and to reduce risk of myocardial infarction and fatal ischaemic heart disease in women [14]. Linoleic acid one plays a crucial role in the diet. Without a source of arachidonic acid or compounds like linoleic acid, which can be converted into arachidonic acid, synthesis of prostaglandins would be compromised, and this would seriously affect many normal metabolic processes [14].
Table 1: mg g-1 total fatty acids (mean ± standard deviation, n=3). ω6/ ω3: omega-6 / omega-3 ratio.

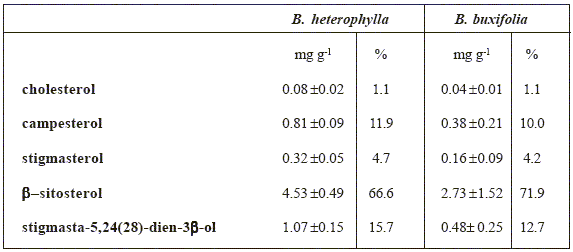
Sterol composition of Berberis seeds are shown in Table 2. Total sterol fractions yield 3.79 (0.4%) and 6.81 (0.7%) mg g-1 total lipids for B. heterophylla and B. buxifolia respectively. In spite of the different total sterol content obtained from our experimental data, the relative percentage of each identified sterol were comparables in both species. All samples contained cholesterol, campesterol, stigmasterol, β-sitosterol, and stigmasta-5,24(28)-dien-3β-ol, with β- sitosterol being predominant in both species, followed by stigmasta-5,24(28)-dien-3β-ol. This last feature is at variance with previous published data in which campesterol occupied the second place of predominance [5]. In the sterol fraction of B. buxifolia, the 3-keto steroid stigmasta 4- en-3-one was also detected. The identification was made via GC/MS and was in agreement with published data [15,16]. This is the first report of the presence of this compound in Berberisgenus.
Table 2: Sterol composition of seed lipids from Berberis species, mg g-1 total lipid, mean ± standard deviation, n = 3, %: relative percentage of the identified sterols.
Conclusion
The information obtained shows that both species of Berberis, B. heterophyla and B. buxifolia, are a good source of high quality fatty acids, when their fruits are wholly consumed. The lipid contents are very interesting, having an important fraction of unsaturated fatty acids which give proven benefits to human health when taken as part of a normal diet. Since all the Berberis species studied serve as food for human and animals, our results may contribute to the knowledge of the nutritional characteristics of these seeds.
References
[1] Correa, M.N. Flora patagónica VIII. Parte IVa, Ed by INTA: Buenos Aires, pp 330- 340, 1984. [ Links ]
[2] Ladio, A.H. Econ. Bot. 2001, 55, 243. [ Links ]
[3] Rapoport E.; Ladio A.; Sanz E. "Plantas nativas comestibles de la Patagonia Andina", partes I y II. Ed. Imaginaria, 1999 and 2003. [ Links ]
[4] Ratera, E.L.; Ratera, M.O. Plantas de la flora argentina empleadas en medicina popular. Hemisferio sur: Buenos Aires, 102, 1980. [ Links ]
[5] Sztarker, N.D.; Cattaneo, P. Ann. Asoc. Quím. Arg. 1976, 64, 281. [ Links ]
[6] Pelliza, A.; Willems, P.; Nakamatsu, V.; Manro, A. Atlas dietario de herbívoros patagónicos, Ed. by R Somlo. Prodesar - INTA-GTZ: Bariloche 23-54, 1997. [ Links ]
[7] Mazzuca, M.; Balzaretti, V.T. J. Sci. Food Agric. 2003, 83, 1072. [ Links ]
[8] Mazzuca, M.; Kraus, W.; Balzaretti, V.T. Journal of Herbal Pharmacotherapy 2003, 3, 31. [ Links ]
[9] Lepage, G.; Roy, C.C.J. Lipid. Res. 1983, 27,114. [ Links ]
[10] Carreras, M.E.; Fuentes, E.; Guzmán, C.A. Biochem. Syst. Ecol.,1989,17,287. [ Links ]
[11] Christie, W.W. Gas chromatography and lipids. The Oily Press: Scotland, pp. 19, 1989. [ Links ]
[12] Simopoulos, A.P. Biomed Pharmacother 2002, 56, 365. [ Links ]
[13] Bjerve, K.S.; Mostad, I.L.; Thoresen, L. Am. J. Clin. Nutr.1987, 45, 66. [ Links ]
[14] Tapiero, H.; Nguyen, Ba. G., Couvreur, P., Tew, K.D. Biomed. Pharmacother, 2002, 56, 215. [ Links ]
[15] Iribarren, A.M. Estudio químico de Bauhinia candicans Bent. Tesis doctoral, FCEN: Universidad de Buenos Aires 82-87, 1984. [ Links ]
[16] Brown, F.J.; Djerassi, C. J. Org. Chem. 1981, 46, 954. [ Links ]




![Preparation, Spectroscopic And Thermal Studies Of The Bridged Isocyanato Complex [Ni2(NCO)2(H2O)4]X2, (X= ½SO4 2− Or NO3− )](/img/es/prev.gif)









